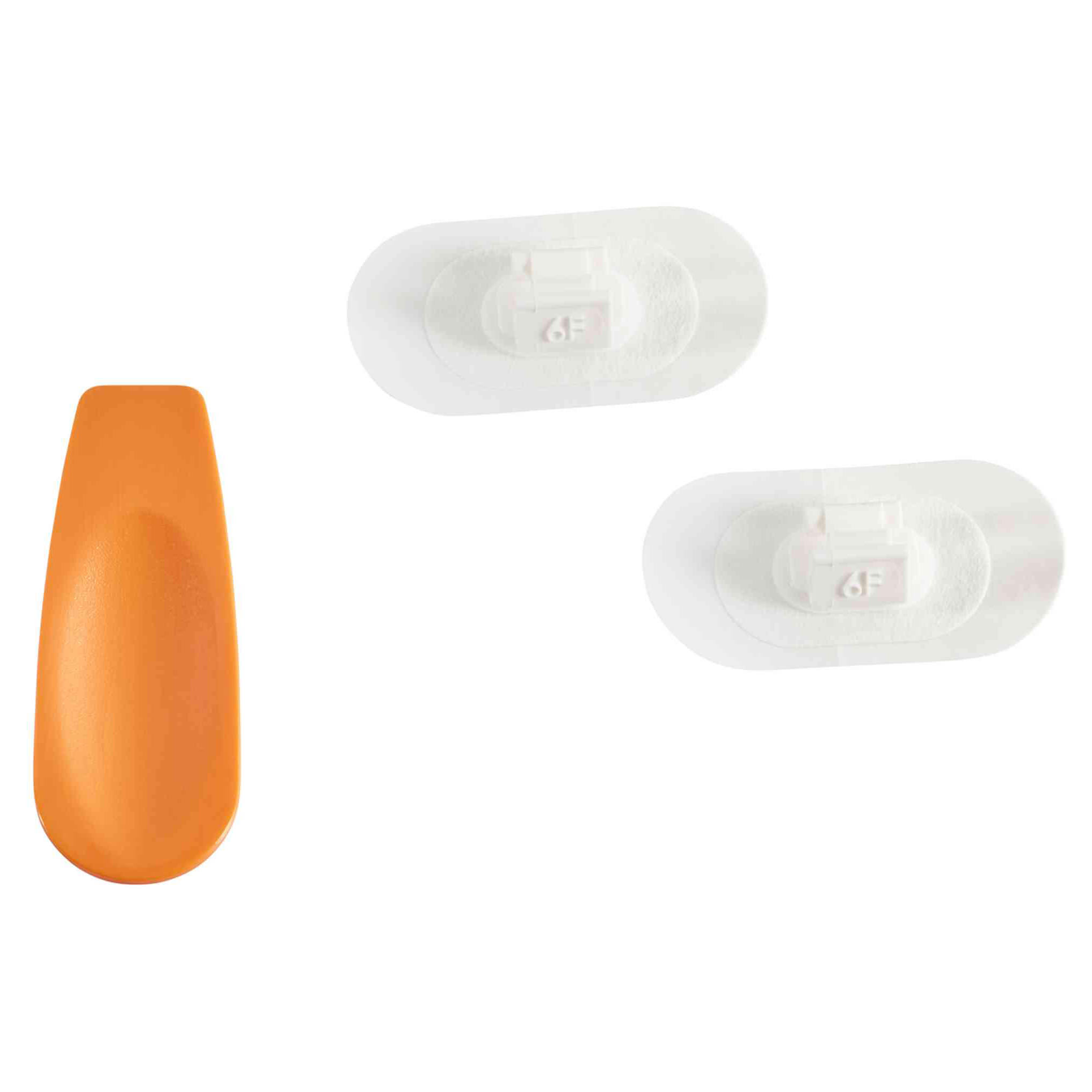
Avanos Ancoris Tube Anchor Set is a secure and easy-to-use solution for stabilizing NG and NI feeding tubes. Designed for both pediatric and adult patients, it helps maintain consistent tube placement while supporting patient comfort and reducing skin irritation risk.
Features
- Approved for pediatric and adult patients.
- Specifically designed clamp to secure the feeding tube
- Secures nasoenteric feeding tubes for an average of three days.
- A smaller skin contact area may lower the risk of skin irritation.
- Compatible with the most popular tiny-bore feeding tubes on the market.
- The clamp enables tube adjustment without changing the complete apparatus.
- A unique clamp and extended-wear adhesive are designed to keep tubes secure.
- Flexible location on the face allows the practitioner to secure the tube away from the patient's line of sight.
- The small size clamp enables for excellent view of the tube centimeter marks, which helps monitor for tube migration.
Kit Contents
- Ancoris Tube Anchors (2)
- Ancoris Opening Tool (1)
- User Guide
Indication for Use / Intended Purpose
Avanos Ancoris Tube Anchor Set is a single-use medical device for externally securing tubes ranging from 6 Fr to 14 Fr in pediatric and adult patients. The gadget is meant for use by or under the supervision of skilled physicians and professional caregivers.
Contraindications
The Ancoris Tube Anchor Set is not recommended for patients who may tug on their tube or anchor(s) excessively, resulting in tube dislodgement or self-injury. Should a patient be likely, based on clinical condition or mental status, to repeatedly pull on the tube or ANCORIS* Tube Anchors, it is recommended to secure the tube via alternate means to minimize potential injury or tube displacement. This device is contraindicated for placement anywhere on patients who have known skin allergies to any of the device materials, or directly onto or in proximity to skin that is burned or irritated, or that has any type of skin lesion(s). This product is contraindicated for direct placement on neonates and/or on patients with fragile skin. Should redness or other signs of irritation appear, remove the device. If conditions persist, consult a physician.
Caution: Use of an adhesive securement device may result in patient discomfort or skin irritation. Infrequently occurring factors arising from the use or misuse of securement devices include: allergic reaction, contamination, delay in nutrition or medication, or the need for additional medical procedures.
Warning
Do not reuse or reprocess these medical devices. Reuse or reprocessing may
- Adversely affect the known biocompatibility of the device
- Compromising the structural integrity of the device
- Lead to the device not performing as intended, or
- Create a risk of contamination and cause the transmission of infectious diseases, resulting in a patient injury, illness, or death.