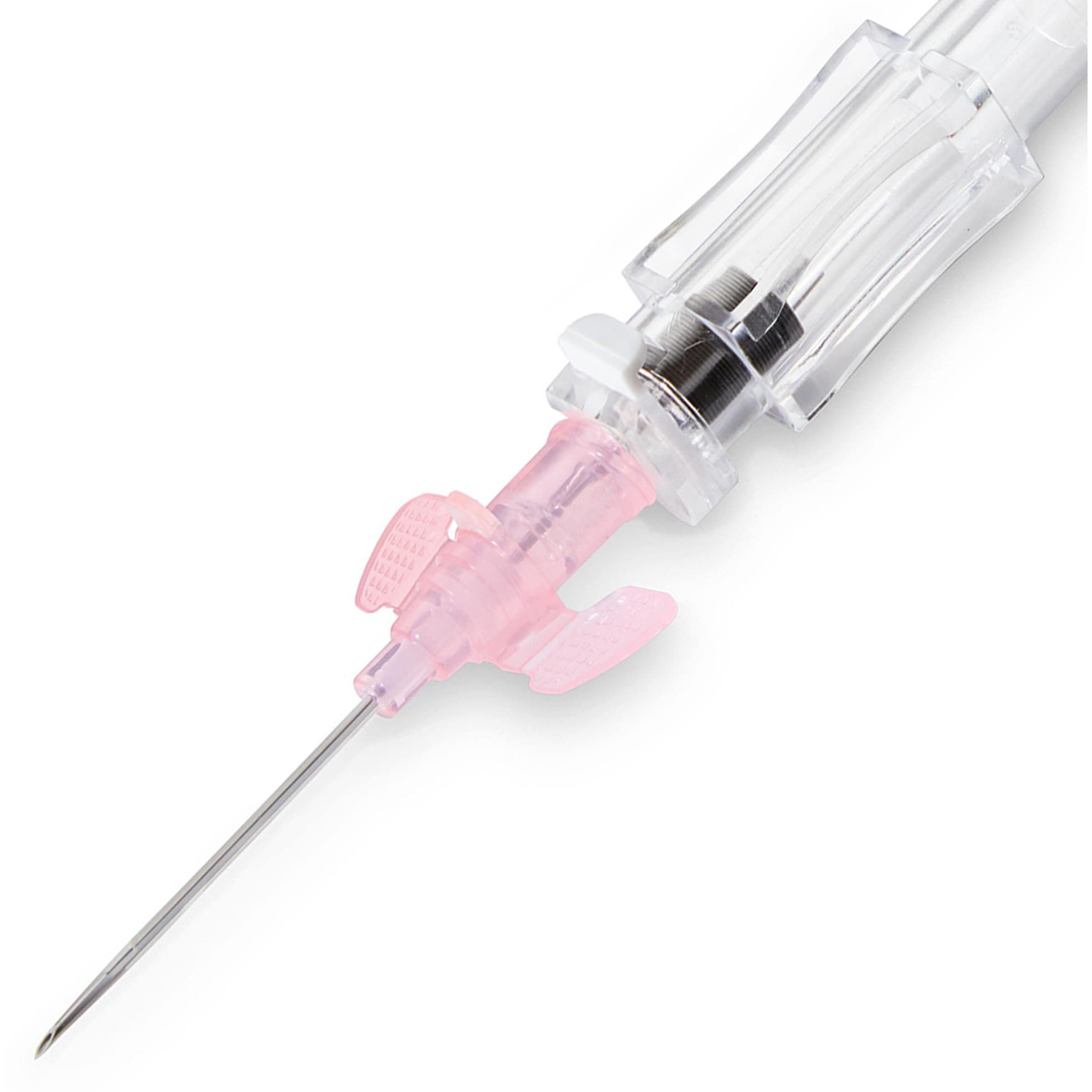
BD Insyte Autoguard BC Peripheral IV Catheter with Winged Hub
Starts From
Description
Confidently keep blood in its place—away from you and your patients.
IV catheter placement is the most common invasive hospital procedure worldwide.1,2 Up to 90% of hospitalized patients require IV therapy, over 95% of these devices are peripheral IV catheters (PIVC). With every PIVC insertion attempt, healthcare workers (HCWs) risk exposure to patient blood.3-5 Needlestick injuries (NSIs) and blood leakage may expose HCWs to more than 30 dangerous pathogens that can be present in human blood, including hepatitis B, C and HIV.6 The BD Insyte Autoguard BC Shielded IV Catheter with blood control technology is designed to provide HCWs protection from needlestick injuries and blood exposure.
Features
- Hub Type: Winged Hub
- Catheter Material: BD Vialon
- Shielded IV catheter with Blood Control Technology
- Push-button shielding technology proven to reduce needlestick injuries
| Model No: | Catheter Length | Color Code | Flow Rate | Gauge | Tubing Diameter | Pressure Rating |
| 382657 | 1.77 Inch | Gray | 195 mL / min | 16 Gauge | 1.3208 to 1.3970 mm ID | Not Pressure Rated |
| 382654 | 1.16 Inch | Gray | 183 mL / min | 16 Gauge | 1.3208 to 1.3970 mm ID | Not Pressure Rated |
| 382647 | 1.88 Inch | Green | 87 mL / min | 18 Gauge | 0.9398 to 1.0160 mm ID | 300 psi |
| 382644 | 1.16 Inch | Green | 95 mL / min | 18 Gauge | 0.9398 to 1.0160 mm ID | 300 psi |
| 382637 | 1.88 Inch | Pink | 54 mL / min | 20 Gauge | 0.7620 to 0.8382 mm ID | 300 psi |
| 382634 | 1.16 Inch | Pink | 61 mL / min | 20 Gauge | 0.7620 to 0.8382 mm ID | 300 psi |
| 382633 | 1 Inch | Pink | 63 mL / min | 20 Gauge | 0.7620 to 0.8382 mm ID | 300 psi |
| 382623 | 1 Inch | Blue | 37 mL / min | 22 Gauge | 0.6096 to 0.6858 mm ID | 300 psi |
| 382612 | 3/4 Inch | Yellow | 20 mL / min | 24 Gauge | 0.4826 to 0.5588 mm ID | Not Pressure Rated |
Specifications
| Brand | Insyte Autoguard BC |
| Manufacturer | BD |
| Application | Peripheral IV Catheter |
| Blood Control | Blood Control |
| Catheter Material | BD Vialon |
| HCPCS | C1769 |
| Hub Material | Plastic Hub |
| Hub Type | Winged Hub |
| Needle Material | Stainless Steel |
| Needle Point Style | Notched Needle |
| Number of Ports | Without Port |
| Port Type | Without Port |
| UNSPSC Code | 42221512 |
| Safety Activation | Push Button Safety |
| Safety Feature | Shielding |
| Sterility | Sterile |
| Usage | Disposable |
| Vessel Entry | Confirmation BD Instaflash Needle |
| X-Ray Compatibility | Radiopaque |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
Confidently keep blood in its place—away from you and your patients.
IV catheter placement is the most common invasive hospital procedure worldwide.1,2 Up to 90% of hospitalized patients require IV therapy, over 95% of these devices are peripheral IV catheters (PIVC). With every PIVC insertion attempt, healthcare workers (HCWs) risk exposure to patient blood.3-5 Needlestick injuries (NSIs) and blood leakage may expose HCWs to more than 30 dangerous pathogens that can be present in human blood, including hepatitis B, C and HIV.6 The BD Insyte Autoguard BC Shielded IV Catheter with blood control technology is designed to provide HCWs protection from needlestick injuries and blood exposure.
Features
- Hub Type: Winged Hub
- Catheter Material: BD Vialon
- Shielded IV catheter with Blood Control Technology
- Push-button shielding technology proven to reduce needlestick injuries
| Model No: | Catheter Length | Color Code | Flow Rate | Gauge | Tubing Diameter | Pressure Rating |
| 382657 | 1.77 Inch | Gray | 195 mL / min | 16 Gauge | 1.3208 to 1.3970 mm ID | Not Pressure Rated |
| 382654 | 1.16 Inch | Gray | 183 mL / min | 16 Gauge | 1.3208 to 1.3970 mm ID | Not Pressure Rated |
| 382647 | 1.88 Inch | Green | 87 mL / min | 18 Gauge | 0.9398 to 1.0160 mm ID | 300 psi |
| 382644 | 1.16 Inch | Green | 95 mL / min | 18 Gauge | 0.9398 to 1.0160 mm ID | 300 psi |
| 382637 | 1.88 Inch | Pink | 54 mL / min | 20 Gauge | 0.7620 to 0.8382 mm ID | 300 psi |
| 382634 | 1.16 Inch | Pink | 61 mL / min | 20 Gauge | 0.7620 to 0.8382 mm ID | 300 psi |
| 382633 | 1 Inch | Pink | 63 mL / min | 20 Gauge | 0.7620 to 0.8382 mm ID | 300 psi |
| 382623 | 1 Inch | Blue | 37 mL / min | 22 Gauge | 0.6096 to 0.6858 mm ID | 300 psi |
| 382612 | 3/4 Inch | Yellow | 20 mL / min | 24 Gauge | 0.4826 to 0.5588 mm ID | Not Pressure Rated |
Specifications
| Brand | Insyte Autoguard BC |
| Manufacturer | BD |
| Application | Peripheral IV Catheter |
| Blood Control | Blood Control |
| Catheter Material | BD Vialon |
| HCPCS | C1769 |
| Hub Material | Plastic Hub |
| Hub Type | Winged Hub |
| Needle Material | Stainless Steel |
| Needle Point Style | Notched Needle |
| Number of Ports | Without Port |
| Port Type | Without Port |
| UNSPSC Code | 42221512 |
| Safety Activation | Push Button Safety |
| Safety Feature | Shielding |
| Sterility | Sterile |
| Usage | Disposable |
| Vessel Entry | Confirmation BD Instaflash Needle |
| X-Ray Compatibility | Radiopaque |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Starts From