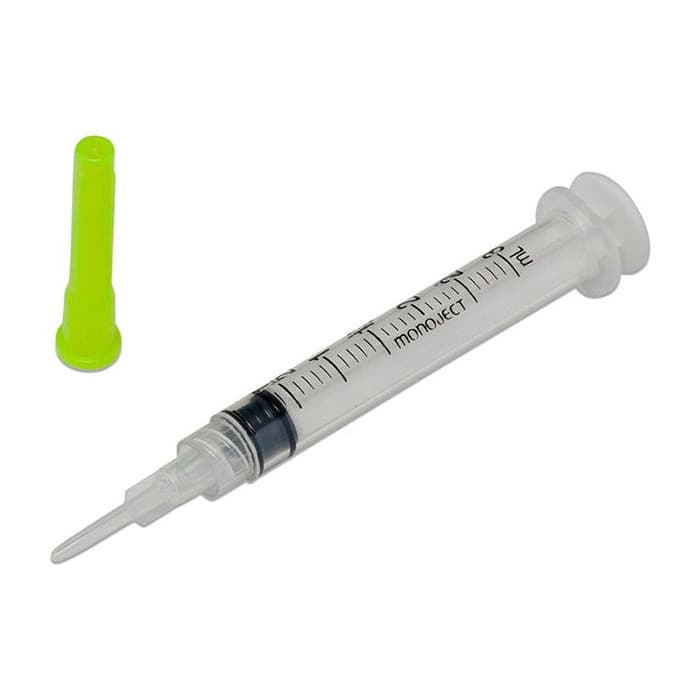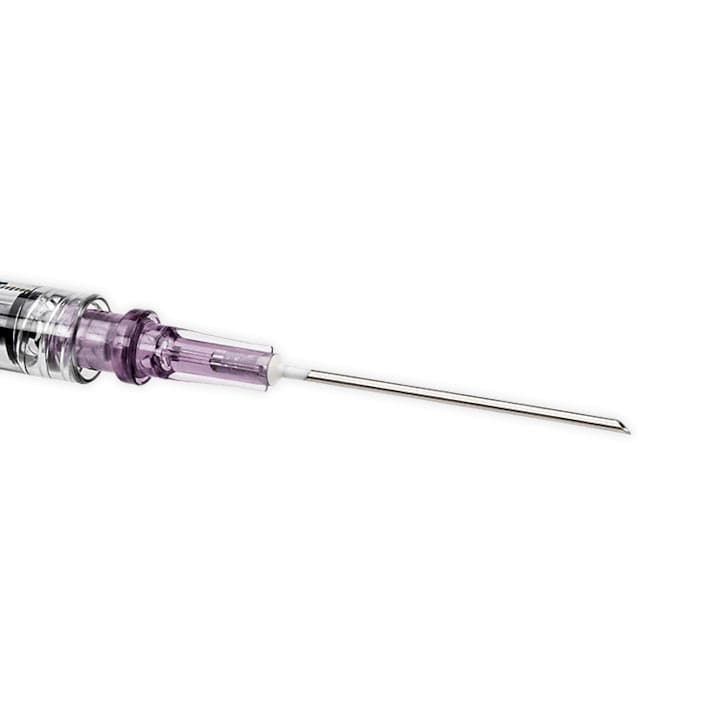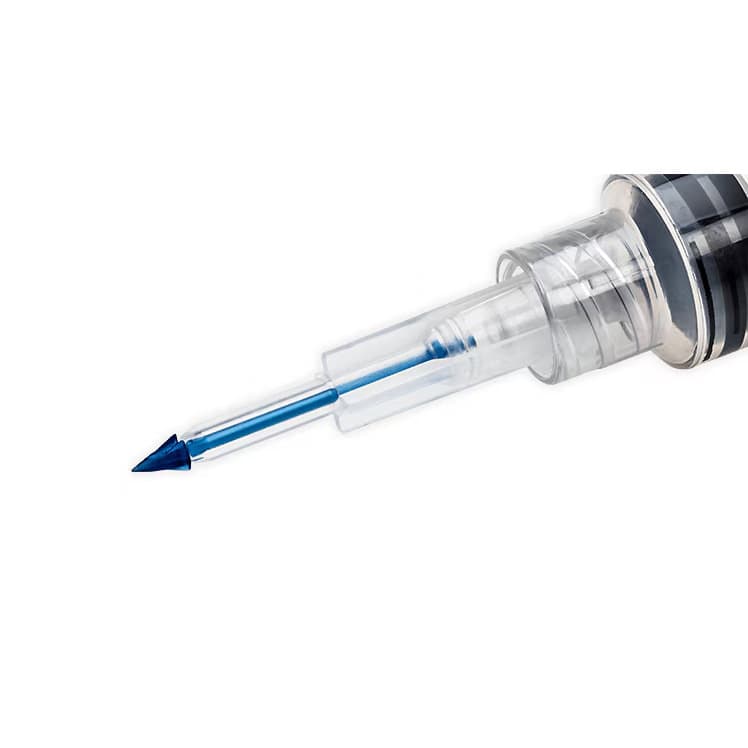

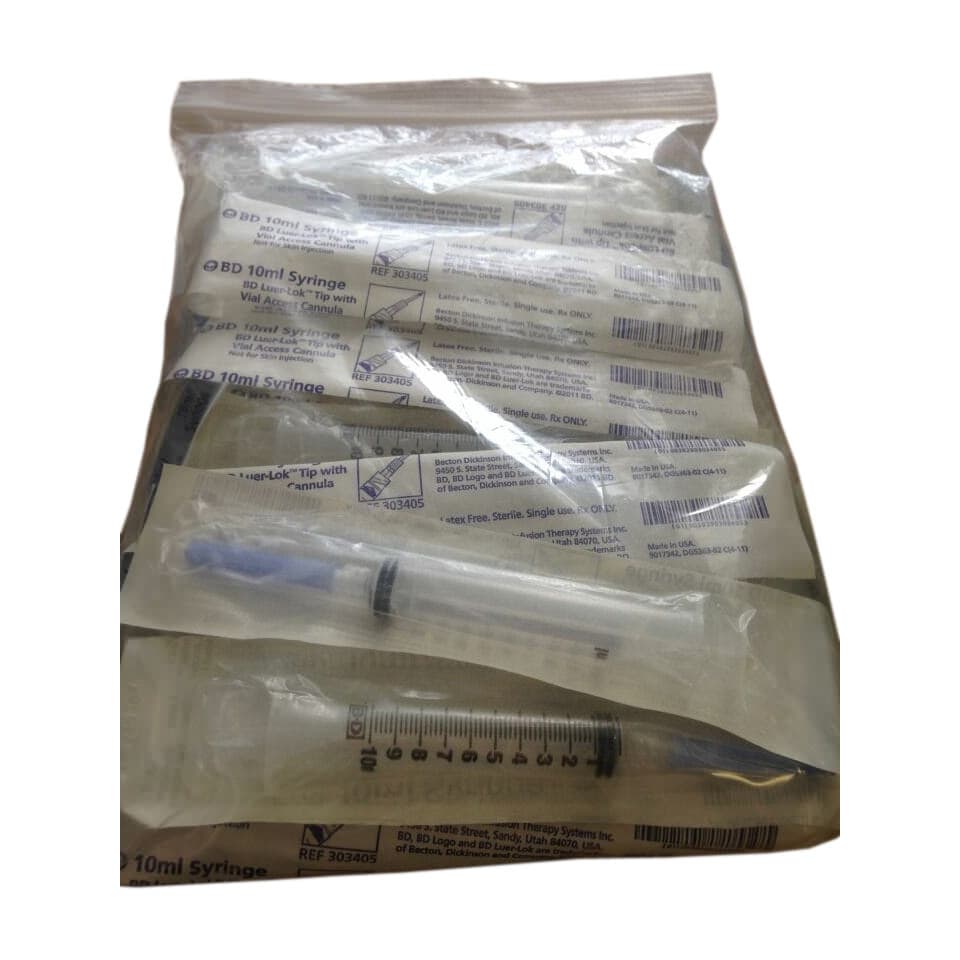

BD Interlink Syringe with Vial Access Cannula, 10mL, 15G, Sterile, Latex-Free
Earn Loyalty Points
+1Buy more to unlock extra rewards!
Description
BD Interlink Syringe with Vial Access Cannula is used for needleless access of a single–dose vial. The BD Interlink Vial Access Cannula are specifically designed for use with Interlink injection sites, identified by a colored alert ring around the septum. Not compatible with conventional injection sites.
Features
- Sterile.
- 15 Gauge
- Latex-free.
- Volume: 10mL
- Needleless system.
Specifications
| Brand | Interlink |
| Manufacturer | BD |
| Application | Cannula with Syringe |
| Gauge | 15 Gauge |
| Graduations | 0.2 cc Increments |
| HCPCS | A4657 |
| Hub Material | Polypropylene Hub |
| Sterility | Sterile |
| Syringe Material | Polypropylene Syringe |
| Type | Vial Access |
| UNSPSC Code | 42221500 |
| Volume | 10 mL |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
BD Interlink Syringe with Vial Access Cannula is used for needleless access of a single–dose vial. The BD Interlink Vial Access Cannula are specifically designed for use with Interlink injection sites, identified by a colored alert ring around the septum. Not compatible with conventional injection sites.
Features
- Sterile.
- 15 Gauge
- Latex-free.
- Volume: 10mL
- Needleless system.
Specifications
| Brand | Interlink |
| Manufacturer | BD |
| Application | Cannula with Syringe |
| Gauge | 15 Gauge |
| Graduations | 0.2 cc Increments |
| HCPCS | A4657 |
| Hub Material | Polypropylene Hub |
| Sterility | Sterile |
| Syringe Material | Polypropylene Syringe |
| Type | Vial Access |
| UNSPSC Code | 42221500 |
| Volume | 10 mL |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.