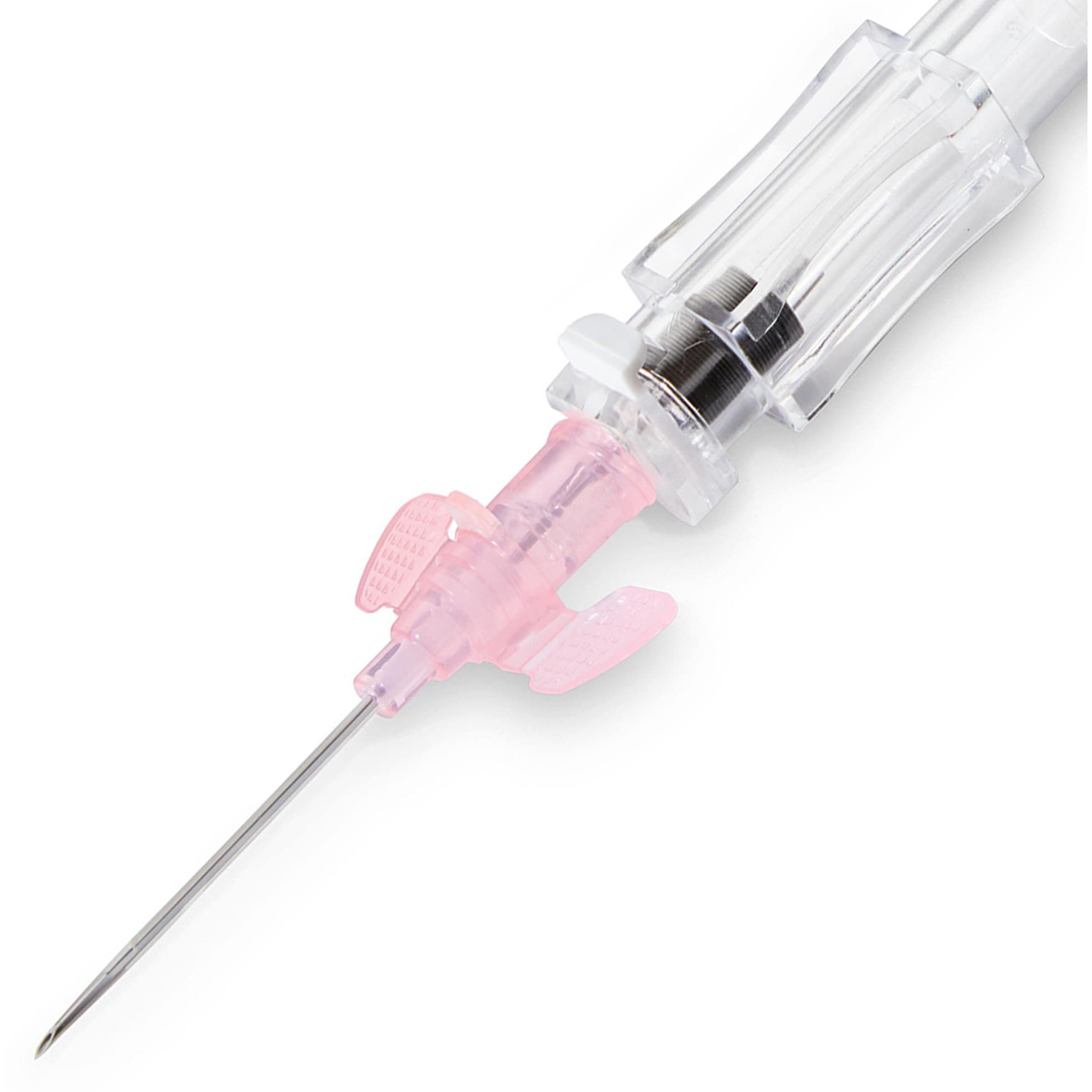
Introcan Safety 2 Closed IV Catheter with One-Time Blood Control, Straight Hub
PayPal Pay Later
Starts From
Description
At B. Braun, developed Introcan Safety 2 with the guiding principle of making the job of IV access safe for the clinician. IV access is an indispensable element of today’s infusion therapy. However, this invasive procedure continues to be associated with potential risks.
Features
- Universal Bevel.
- Passive Safety Needle Shield slides into place as needle is pulled out of catheter: Aids in prevention of needlestick injuries.
- Designed to provide a quick visual confirmation of vein puncture with separate needle/flashback chamber and catheter flashback.
Making Access Safe for Clinicians
Needlestick injuries continue to be one of the highest risks clinicians will face during their daily routine. Studies show that passive, fully automatic safety devices reduce needlestick injury prevention by up to 48%.
With B. Braun’s Introcan Safety 2, you are protected by a truly automatic passive safety device.
Safety for Clinicians
- Deploys automatically
- Cannot be bypassed after needle is removed
- Prevents needle reinsertions when activated
Making Access Efficient
Multiple attempts to establish IV access can lead to a delay in treatment plus an increase in material cost and time.
The Double Flashback Technology of Introcan Safety 2 supports first stick success through quick visual confirmation that both needle and catheter are successfully in the vein.
Making Access Convenient
The One-time Blood Control Septum is designed to restrict the flow of blood from the catheter hub after needle removal until first connection of a luer access device.
Introcan Safety 2's blood control during insertion
- Helps reduce bloodborne pathogen exposure.
- Use of blood control IVCs reduces cleanup time and materials.
Making Access Intuitive
Based on a study, 95% of clinicians found Introcan Safety 2 easy to insert with one hand
- Easy to insert the catheter with one hand.
- Easy to thread the catheter with one hand.
User Benefits
- Passive safety reduces needlestick injuries.
- Minimizes blood exposure during insertion.
Flexibility of Insertion Angles
The universal bevel is designed to allow flexibility of insertion angles.
Specifications
| Brand | Introcan Safety |
| Manufacturer | B. Braun |
| Application | Closed IV Catheter |
| Blood Control | Blood Control |
| Catheter Material | Polyurethane |
| DEHP Indicator | Not made with DEHP |
| Hub Material | Plastic Hub |
| Hub Type | Straight Hub |
| Needle Material | Stainless Steel |
| Needle Point Style | Bevel Needle |
| Number of Ports | Without Port |
| Safety Activation | Passive Safety |
| Safety Feature | Sliding |
| Sterility | Sterile |
| Usage | Disposable |
| Vessel Entry Confirmation | Flashback Chamber / Flashback Catheter |
| X-Ray Compatibility | Radiopaque |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
At B. Braun, developed Introcan Safety 2 with the guiding principle of making the job of IV access safe for the clinician. IV access is an indispensable element of today’s infusion therapy. However, this invasive procedure continues to be associated with potential risks.
Features
- Universal Bevel.
- Passive Safety Needle Shield slides into place as needle is pulled out of catheter: Aids in prevention of needlestick injuries.
- Designed to provide a quick visual confirmation of vein puncture with separate needle/flashback chamber and catheter flashback.
Making Access Safe for Clinicians
Needlestick injuries continue to be one of the highest risks clinicians will face during their daily routine. Studies show that passive, fully automatic safety devices reduce needlestick injury prevention by up to 48%.
With B. Braun’s Introcan Safety 2, you are protected by a truly automatic passive safety device.
Safety for Clinicians
- Deploys automatically
- Cannot be bypassed after needle is removed
- Prevents needle reinsertions when activated
Making Access Efficient
Multiple attempts to establish IV access can lead to a delay in treatment plus an increase in material cost and time.
The Double Flashback Technology of Introcan Safety 2 supports first stick success through quick visual confirmation that both needle and catheter are successfully in the vein.
Making Access Convenient
The One-time Blood Control Septum is designed to restrict the flow of blood from the catheter hub after needle removal until first connection of a luer access device.
Introcan Safety 2's blood control during insertion
- Helps reduce bloodborne pathogen exposure.
- Use of blood control IVCs reduces cleanup time and materials.
Making Access Intuitive
Based on a study, 95% of clinicians found Introcan Safety 2 easy to insert with one hand
- Easy to insert the catheter with one hand.
- Easy to thread the catheter with one hand.
User Benefits
- Passive safety reduces needlestick injuries.
- Minimizes blood exposure during insertion.
Flexibility of Insertion Angles
The universal bevel is designed to allow flexibility of insertion angles.
Specifications
| Brand | Introcan Safety |
| Manufacturer | B. Braun |
| Application | Closed IV Catheter |
| Blood Control | Blood Control |
| Catheter Material | Polyurethane |
| DEHP Indicator | Not made with DEHP |
| Hub Material | Plastic Hub |
| Hub Type | Straight Hub |
| Needle Material | Stainless Steel |
| Needle Point Style | Bevel Needle |
| Number of Ports | Without Port |
| Safety Activation | Passive Safety |
| Safety Feature | Sliding |
| Sterility | Sterile |
| Usage | Disposable |
| Vessel Entry Confirmation | Flashback Chamber / Flashback Catheter |
| X-Ray Compatibility | Radiopaque |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Starts From