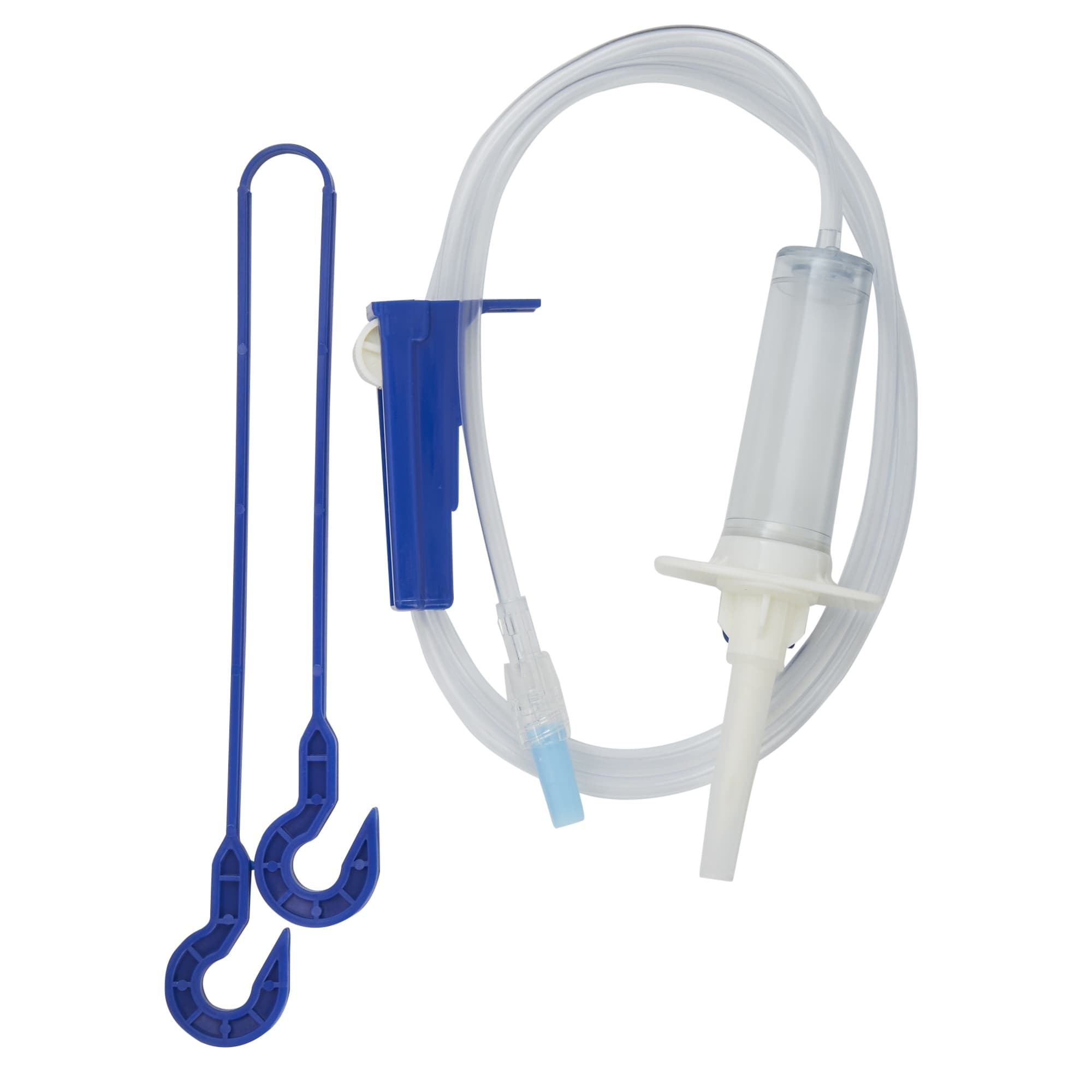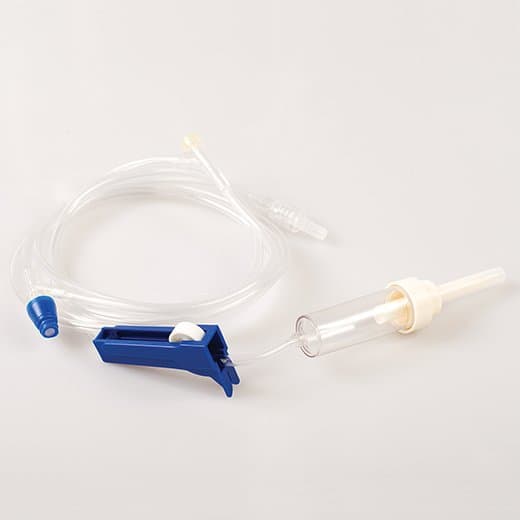
MedSource Gravity Primary IV Administration Set, 10 Drops per mL Drip Rate
Starts From
Description
MedSource IV Administration Sets have a color coded packaging system to enable quick visual identification of necessary drop setting and are available in a variety of configurations to meet specific patient needs.
Specifications
- Non-pyrogenic
- Individually packaged
- Not made with DEHP
- Sterilization method: EO
- Material: Medical grade PVC
- Not made with natural rubber latex
Specifications
| Brand | MedSource |
| Administration Method | Gravity |
| Application | Primary IV Administration Set |
| Back Check Valve | Without Back Check Valve |
| Connection Type | Rotating Male Luer Lock Connector |
| DEHP Indicator | DEHP-Free Tubing |
| Drip Rate | 10 Drops / mL Drip Rate |
| Filter | Without Filter |
| Flow Control Type | Roller Clamp |
| Flow Regulator | Without Flow Regulator |
| HCPCS | S1015 |
| Injection Port Type | Grouping Standard Injection Port |
| Material | Medical Grade PVC, Non-Pyrogenic |
| Number of Ports | 2 Ports |
| Port Type | Split Septum Y-Port |
| Priming Volume | 8 mL Priming Volume |
| Spike Type | Vented Spike |
| Sterility | Sterile |
| Tubing Length | 83 Inch Tubing |
| Type | Solution |
| UNSPSC Code | 42221609 |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
MedSource IV Administration Sets have a color coded packaging system to enable quick visual identification of necessary drop setting and are available in a variety of configurations to meet specific patient needs.
Specifications
- Non-pyrogenic
- Individually packaged
- Not made with DEHP
- Sterilization method: EO
- Material: Medical grade PVC
- Not made with natural rubber latex
Specifications
| Brand | MedSource |
| Administration Method | Gravity |
| Application | Primary IV Administration Set |
| Back Check Valve | Without Back Check Valve |
| Connection Type | Rotating Male Luer Lock Connector |
| DEHP Indicator | DEHP-Free Tubing |
| Drip Rate | 10 Drops / mL Drip Rate |
| Filter | Without Filter |
| Flow Control Type | Roller Clamp |
| Flow Regulator | Without Flow Regulator |
| HCPCS | S1015 |
| Injection Port Type | Grouping Standard Injection Port |
| Material | Medical Grade PVC, Non-Pyrogenic |
| Number of Ports | 2 Ports |
| Port Type | Split Septum Y-Port |
| Priming Volume | 8 mL Priming Volume |
| Spike Type | Vented Spike |
| Sterility | Sterile |
| Tubing Length | 83 Inch Tubing |
| Type | Solution |
| UNSPSC Code | 42221609 |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Starts From