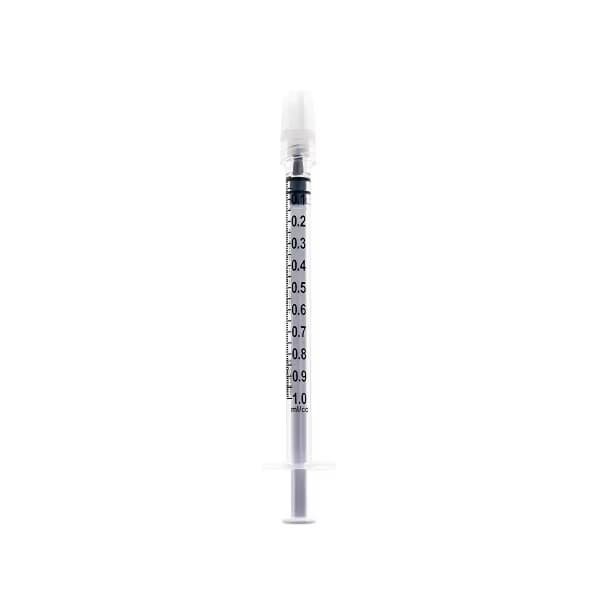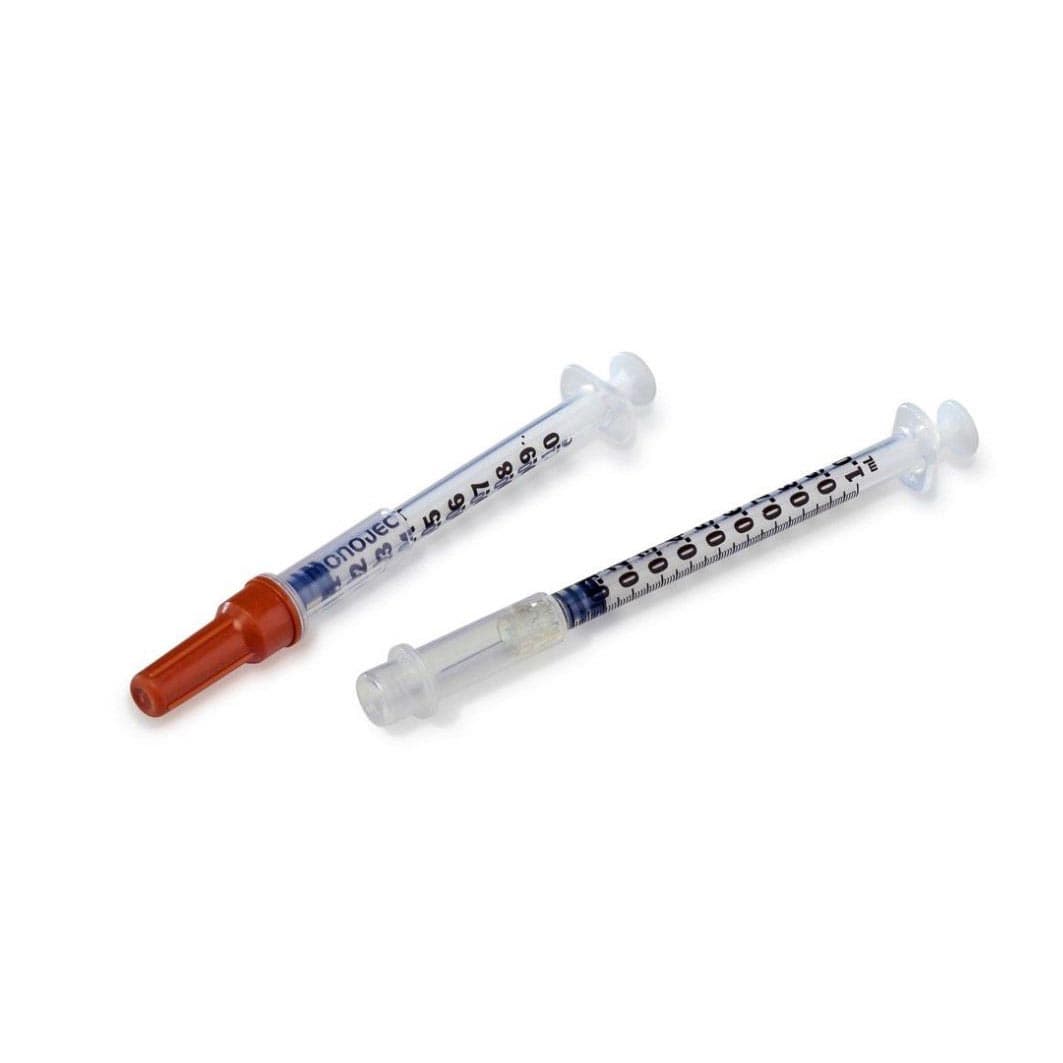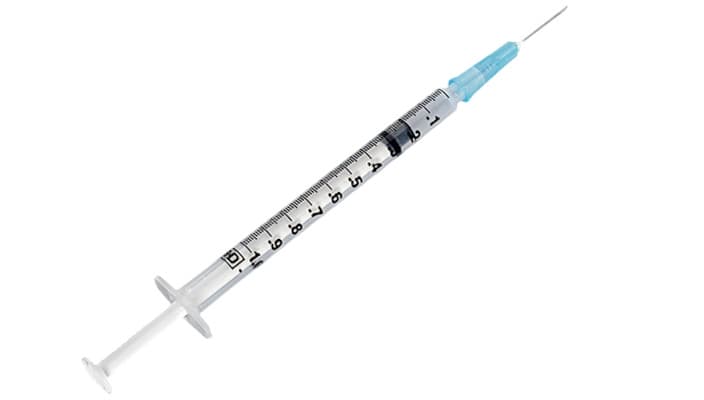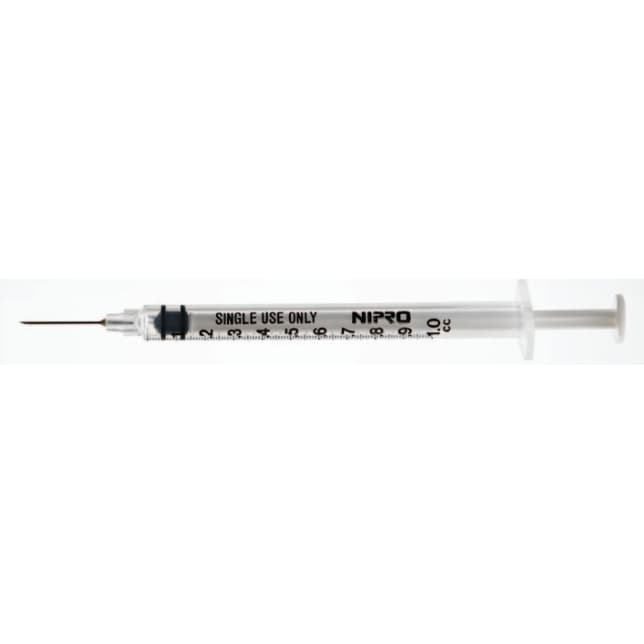
Nipro Standard Tuberculin Syringe with Needle
PayPal Pay Later
Starts From
Description
Nipro manufactures a full range of Tuberculin Syringe to match a variety of clinical needs. State-of-the-art manufacturing assures products of consistently high quality that also meet the most rigorous clinical requirements.
Features
- Materials are inert and compatible with delivered medications
- Syringes are ETO-sterilized and undergo automated inspection to assure uniform quality
- Polypropylene syringe barrel and specially formulated elastomer stopper result in a smooth, accurate motion along the entire length of the barrel
Specifications
| Brand | Nipro Medical |
| Application | Standard Tuberculin Syringe with Needle |
| Needle Material | Medical Grade Stainless Steel Needle |
| Needle Point Style | Regular Bevel |
| Needle Style | Detachable Needle |
| Packaging Type | Blister Pack |
| Safety Feature | NonSafety |
| Sterility | Sterile |
| Syringe Material | Polypropylene Syringe |
| Usage | Disposable |
| Wall Type | Regular Wall |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
Nipro manufactures a full range of Tuberculin Syringe to match a variety of clinical needs. State-of-the-art manufacturing assures products of consistently high quality that also meet the most rigorous clinical requirements.
Features
- Materials are inert and compatible with delivered medications
- Syringes are ETO-sterilized and undergo automated inspection to assure uniform quality
- Polypropylene syringe barrel and specially formulated elastomer stopper result in a smooth, accurate motion along the entire length of the barrel
Specifications
| Brand | Nipro Medical |
| Application | Standard Tuberculin Syringe with Needle |
| Needle Material | Medical Grade Stainless Steel Needle |
| Needle Point Style | Regular Bevel |
| Needle Style | Detachable Needle |
| Packaging Type | Blister Pack |
| Safety Feature | NonSafety |
| Sterility | Sterile |
| Syringe Material | Polypropylene Syringe |
| Usage | Disposable |
| Wall Type | Regular Wall |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Starts From