Starts From
Description
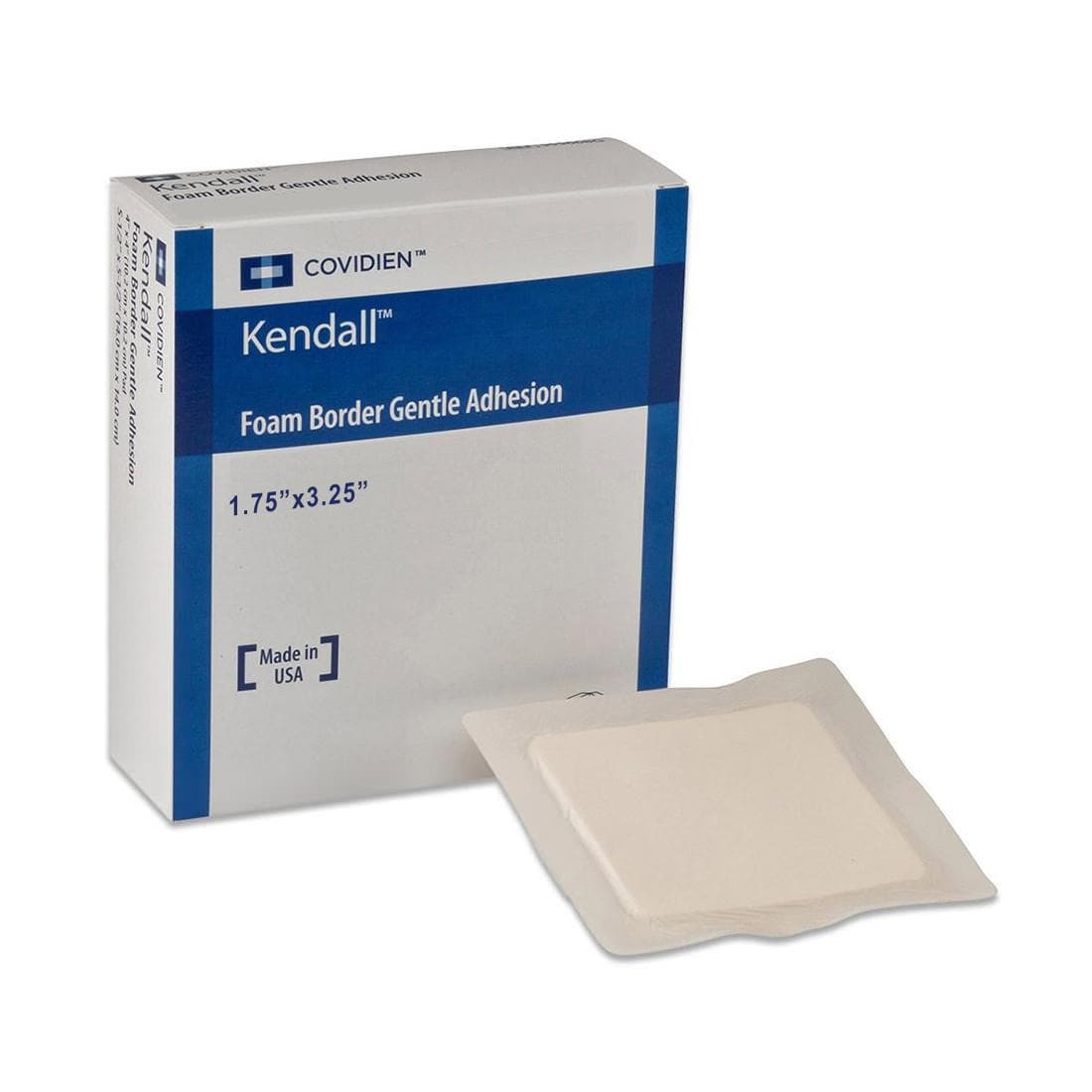
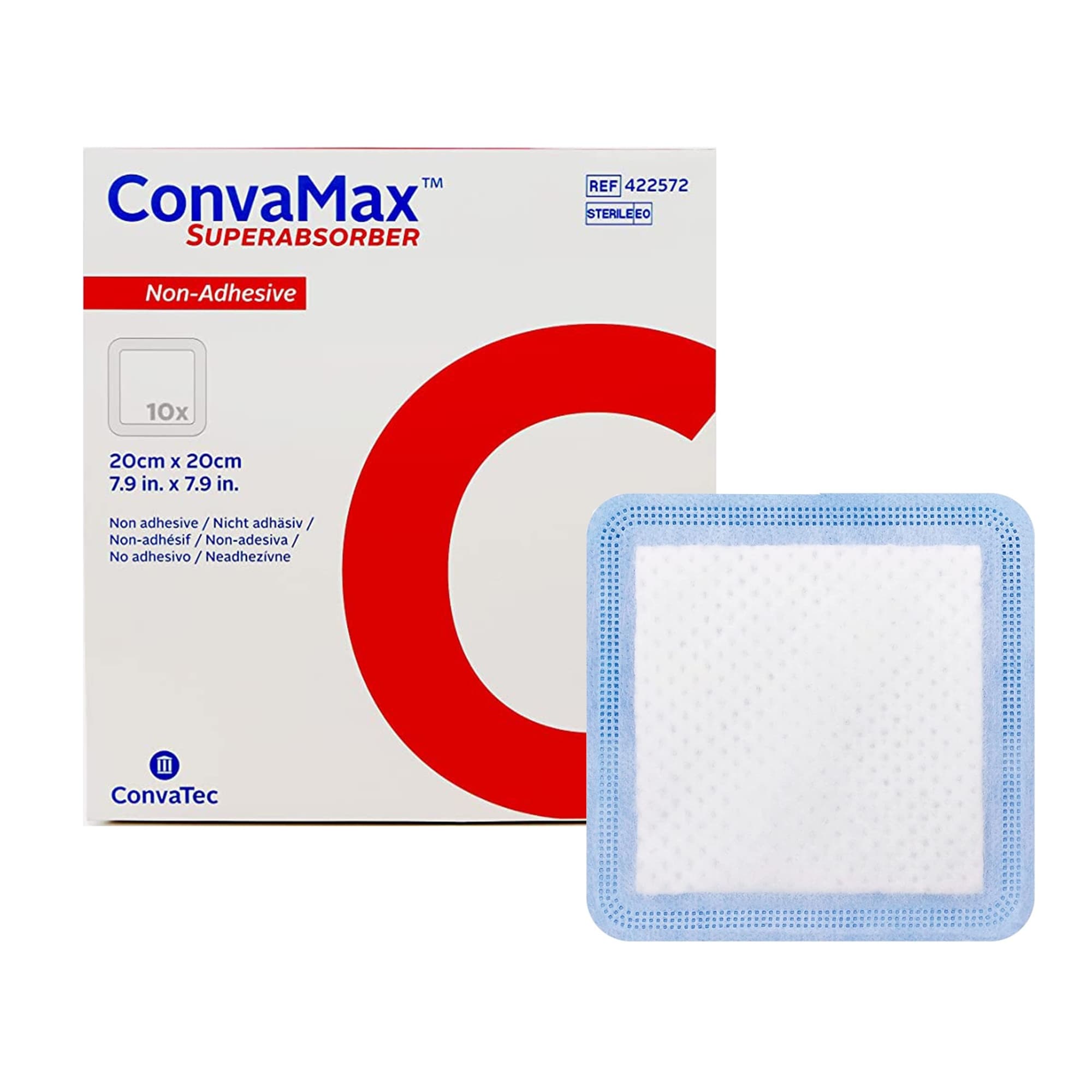
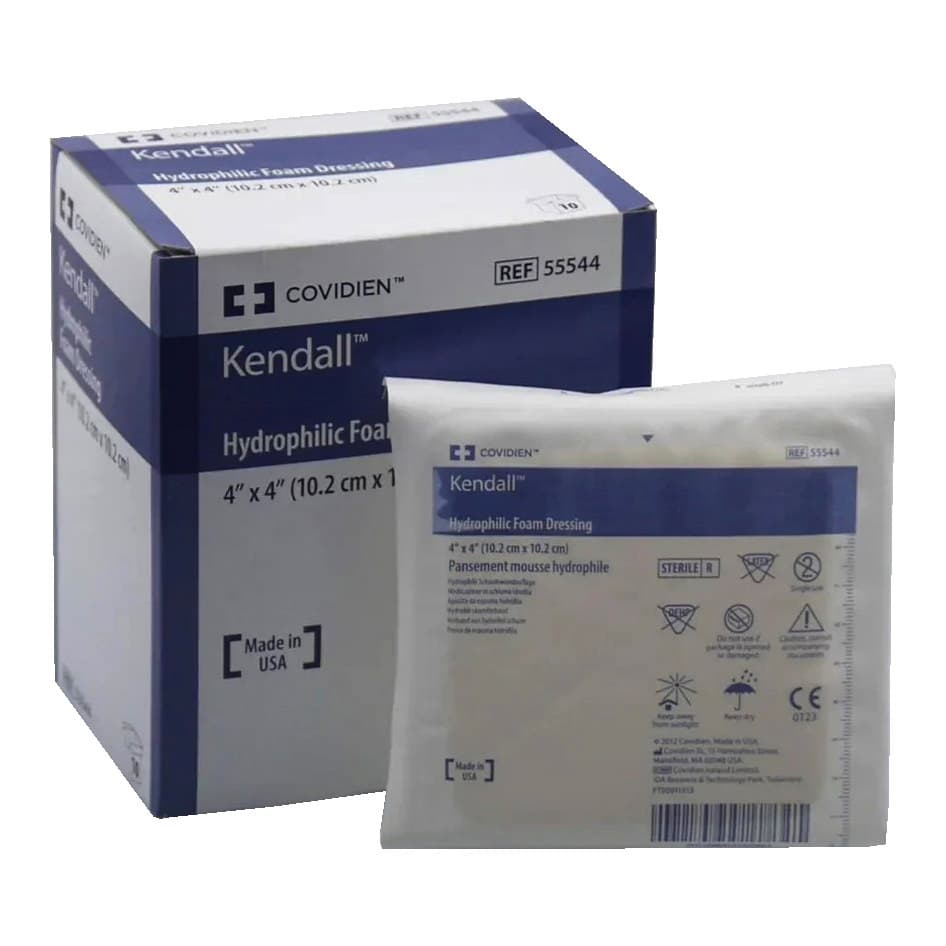
Smith & Nephew Allevyn Non-Adhesive Hydrocellular Polyurethane Dressing has a trilaminate structure which is comprised of a non-Adherent, wound contact layer, a hydrocellular core, and a breathable outer surface. The outer, pink layer allows the formation and maintenance of a moist wound environment at the wound surface, helping to prevent eschar formation. The pink film is also waterproof and aids in the prevention of bacterial contamination.
Features
- Easy to apply and remove.
- Suitable for use under compression bandaging.
- Wide range of sizes that can be cut to suit different body contours.
- Soft and comfortable for the patient and conformable to challenging body areas.
- ALLEVYN Heel can contribute to a pressure relieving protocol when used prophylactically.
- Unique triple action technology effectively manages fluid to create a moist wound environment.
- Highly breathable top film may help to minimize the risk of maceration to the wound and peri-wound.
- The “cup” design of the ALLEVYN Heel Dressing was designed to conform to these awkward areas like heels and elbows.
- Non-adherent wound contact layer means the dressing is suitable for use on fragile skin such as in patients with Epidermolysis Bullosa.
- The backing film provides an effective barrier function to exogenous bacteria as well as helps to prevent fluid strikethrough and assists in preventing bacterial strikethrough.
Indications
- Donor sites.
- Fungating ulcers.
- Infected wounds.
- Surgical wounds.
- Malignant wounds.
- Oncological Wounds.
- Shallow, granulating wounds.
- First and second degree burns.
- Epidermolysis Bullosa wounds.
- Chronic and acute exudative wounds.
- Full- and partial-thickness wounds such as pressure ulcers, leg ulcers and diabetic foot ulcers.
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
Smith & Nephew Allevyn Non-Adhesive Hydrocellular Polyurethane Dressing has a trilaminate structure which is comprised of a non-Adherent, wound contact layer, a hydrocellular core, and a breathable outer surface. The outer, pink layer allows the formation and maintenance of a moist wound environment at the wound surface, helping to prevent eschar formation. The pink film is also waterproof and aids in the prevention of bacterial contamination.
Features
- Easy to apply and remove.
- Suitable for use under compression bandaging.
- Wide range of sizes that can be cut to suit different body contours.
- Soft and comfortable for the patient and conformable to challenging body areas.
- ALLEVYN Heel can contribute to a pressure relieving protocol when used prophylactically.
- Unique triple action technology effectively manages fluid to create a moist wound environment.
- Highly breathable top film may help to minimize the risk of maceration to the wound and peri-wound.
- The “cup” design of the ALLEVYN Heel Dressing was designed to conform to these awkward areas like heels and elbows.
- Non-adherent wound contact layer means the dressing is suitable for use on fragile skin such as in patients with Epidermolysis Bullosa.
- The backing film provides an effective barrier function to exogenous bacteria as well as helps to prevent fluid strikethrough and assists in preventing bacterial strikethrough.
Indications
- Donor sites.
- Fungating ulcers.
- Infected wounds.
- Surgical wounds.
- Malignant wounds.
- Oncological Wounds.
- Shallow, granulating wounds.
- First and second degree burns.
- Epidermolysis Bullosa wounds.
- Chronic and acute exudative wounds.
- Full- and partial-thickness wounds such as pressure ulcers, leg ulcers and diabetic foot ulcers.
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Starts From