




B. Braun Introcan Safety Peripheral IV Catheter, FEP Polymer
Starts From
Description
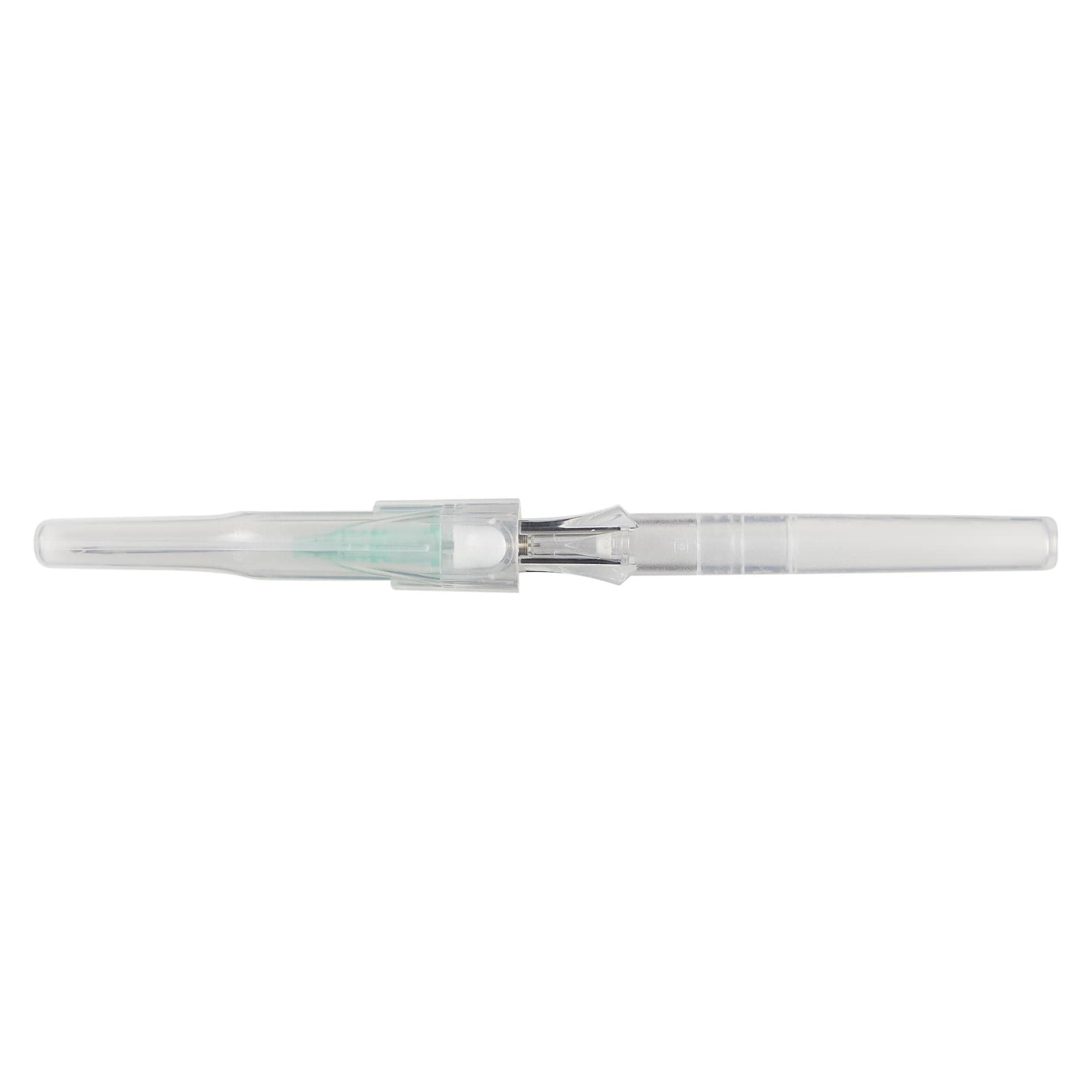
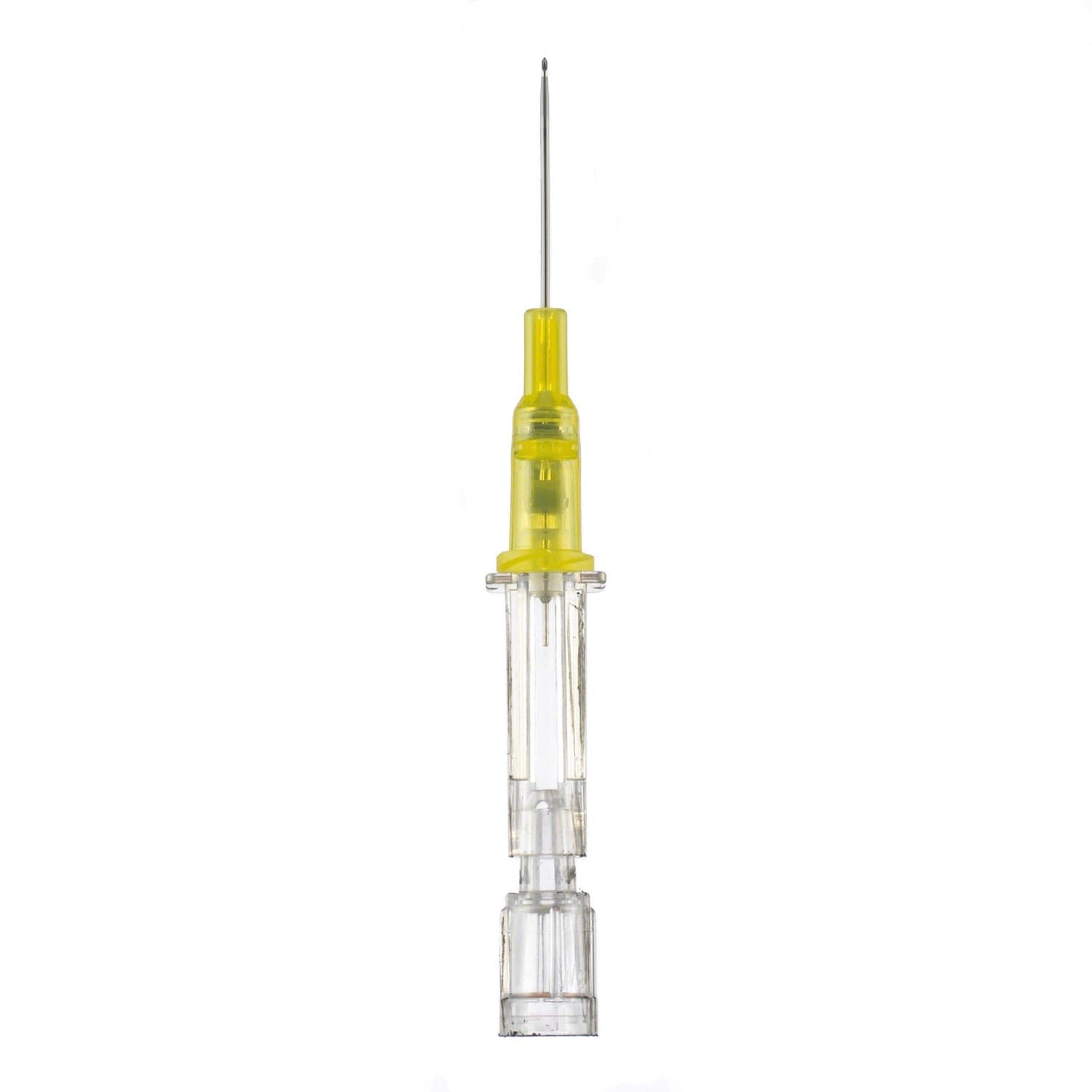
B. Braun Introcan Safety Peripheral IV Catheter comes with a passive safety device that is automatically activated and cannot be bypassed. Easy to use and features double flashback technology for successful needle placement every time.
Although safety devices were introduced over a decade ago, preventing needlestick injuries continues to be a major concern. With the B. Braun Introcan Safety IV Catheter, you are protected by a truly passive safety device that is activated automatically and cannot be by-passed. No extra steps are required to ensure healthcare worker and patient safety.
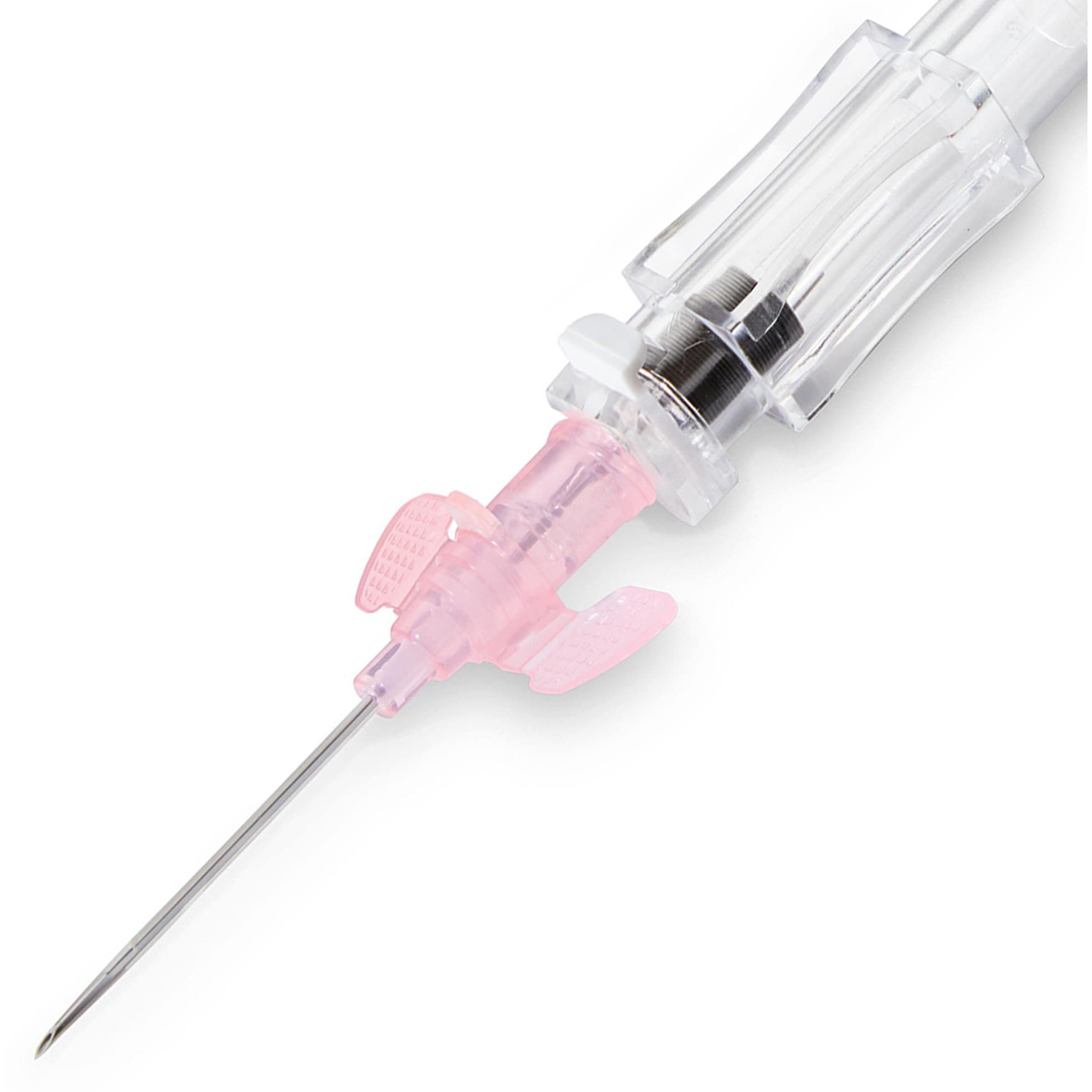
This easy-to-use safety IV catheter incorporates separate needle and catheter flashback - Double Flashback technology - that gives you the added confidence of successful placement every time. The sharp needle features a Universal Bevel geometry that allows a wider choice of insertion angles, which is designed to make the catheter easy to adapt to and venipuncture more comfortable for patients. And with less plastic content than other safety IV catheters, you can potentially reduce storage space and medical waste.
Features
Raise your facility’s standard of care and choose the B. Braun Introcan Safety IV Catheter to:
- Help the Environment: lighter, more compact design can potentialy lower shipping, storage and waste disposal costs.
- Improve Patient Comfort: the sharp needle features a Universal Bevel that is designed for a wider choice of insertion angles and produces less tearing.
- Reduce Needlestick Injuries: recent studies, confirm that passive safety designs offer 2-3 times better protection against accidental needlesticks than active designs. INS Standards also recommend the use of passive safety devices.
- Ensure Best Practices: the passive safety design ensures that the safety mechanism is always activated and prevents re-insertion of the stylet. Additionally, a recent study shows Introcan Safety results in less blood exposure to staff than catheters featuring spring-retraction of the needle.
- Promote First Stick Success: the innovative Double Flashback technology allows separate confirmation of both needle and catheter placement, which can result in better first stick success and greater clinician confidence. New technology in our 24G catheters is designed to speed flashback of blood to help confirm placement in patients. Research also shows Introcan Safety® is easier to introduce into the vein compared to the leading IV catheter.
Specifications
| Brand | Introcan Safety |
| Manufacturer | B. Braun |
| Application | Peripheral IV Catheter |
| Catheter Material | FEP Polymer |
| Hub Material | Plastic Hub |
| Hub Type | Straight Hub |
| Needle Material | Stainless Steel |
| Usage | Disposable |
| Needle Point Style | Universal Bevel |
| Number of Ports | Without Port |
| Port Type | Without Ports Type |
| Pressure Rating | 300 psi |
| Safety Feature | Sliding Safety Needle |
| Sterility | Sterile |
| X-Ray Compatibility | Radiopaque |
| Catheter Material | FEP Polymer |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
B. Braun Introcan Safety Peripheral IV Catheter comes with a passive safety device that is automatically activated and cannot be bypassed. Easy to use and features double flashback technology for successful needle placement every time.
Although safety devices were introduced over a decade ago, preventing needlestick injuries continues to be a major concern. With the B. Braun Introcan Safety IV Catheter, you are protected by a truly passive safety device that is activated automatically and cannot be by-passed. No extra steps are required to ensure healthcare worker and patient safety.
This easy-to-use safety IV catheter incorporates separate needle and catheter flashback - Double Flashback technology - that gives you the added confidence of successful placement every time. The sharp needle features a Universal Bevel geometry that allows a wider choice of insertion angles, which is designed to make the catheter easy to adapt to and venipuncture more comfortable for patients. And with less plastic content than other safety IV catheters, you can potentially reduce storage space and medical waste.
Features
Raise your facility’s standard of care and choose the B. Braun Introcan Safety IV Catheter to:
- Help the Environment: lighter, more compact design can potentialy lower shipping, storage and waste disposal costs.
- Improve Patient Comfort: the sharp needle features a Universal Bevel that is designed for a wider choice of insertion angles and produces less tearing.
- Reduce Needlestick Injuries: recent studies, confirm that passive safety designs offer 2-3 times better protection against accidental needlesticks than active designs. INS Standards also recommend the use of passive safety devices.
- Ensure Best Practices: the passive safety design ensures that the safety mechanism is always activated and prevents re-insertion of the stylet. Additionally, a recent study shows Introcan Safety results in less blood exposure to staff than catheters featuring spring-retraction of the needle.
- Promote First Stick Success: the innovative Double Flashback technology allows separate confirmation of both needle and catheter placement, which can result in better first stick success and greater clinician confidence. New technology in our 24G catheters is designed to speed flashback of blood to help confirm placement in patients. Research also shows Introcan Safety® is easier to introduce into the vein compared to the leading IV catheter.
Specifications
| Brand | Introcan Safety |
| Manufacturer | B. Braun |
| Application | Peripheral IV Catheter |
| Catheter Material | FEP Polymer |
| Hub Material | Plastic Hub |
| Hub Type | Straight Hub |
| Needle Material | Stainless Steel |
| Usage | Disposable |
| Needle Point Style | Universal Bevel |
| Number of Ports | Without Port |
| Port Type | Without Ports Type |
| Pressure Rating | 300 psi |
| Safety Feature | Sliding Safety Needle |
| Sterility | Sterile |
| X-Ray Compatibility | Radiopaque |
| Catheter Material | FEP Polymer |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Starts From