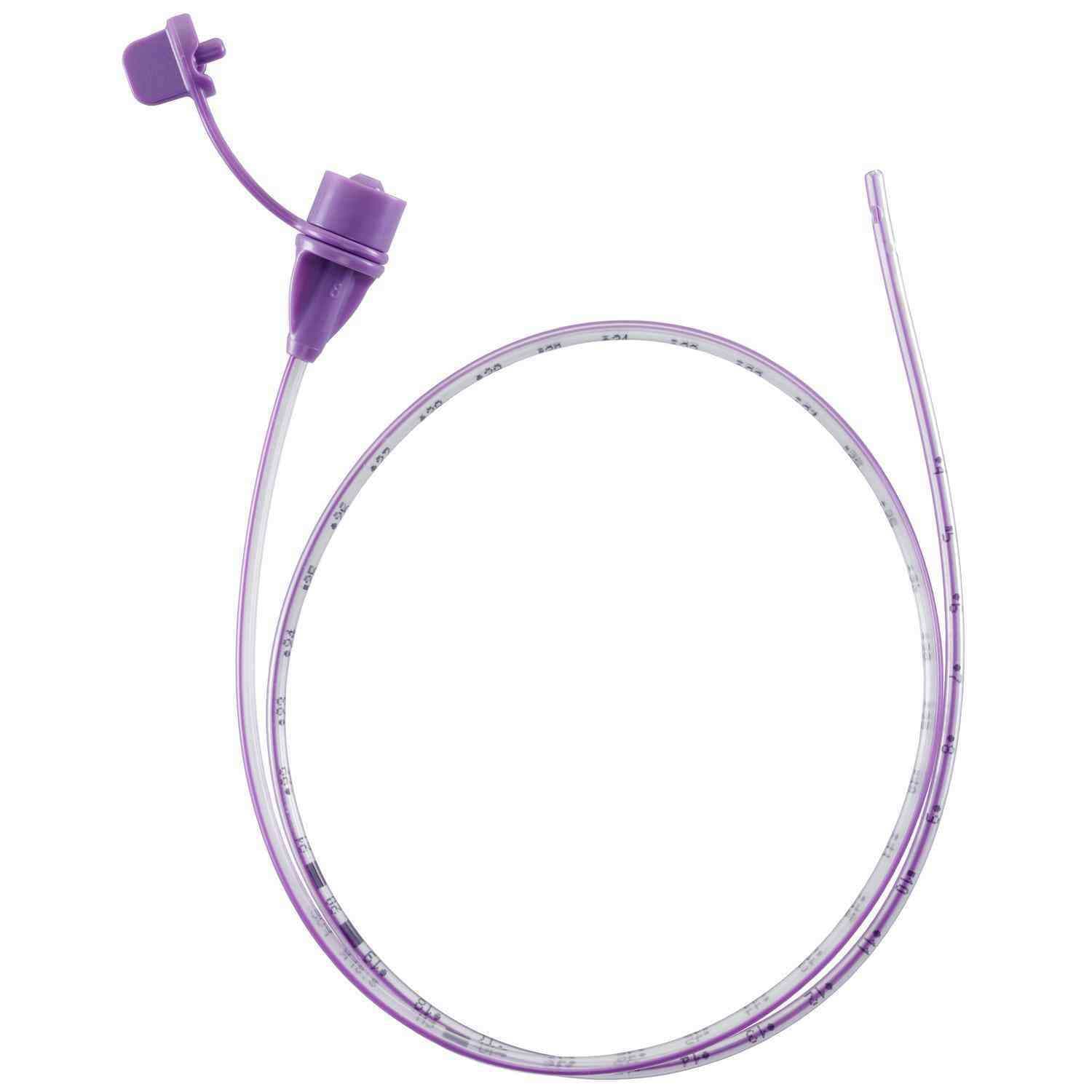
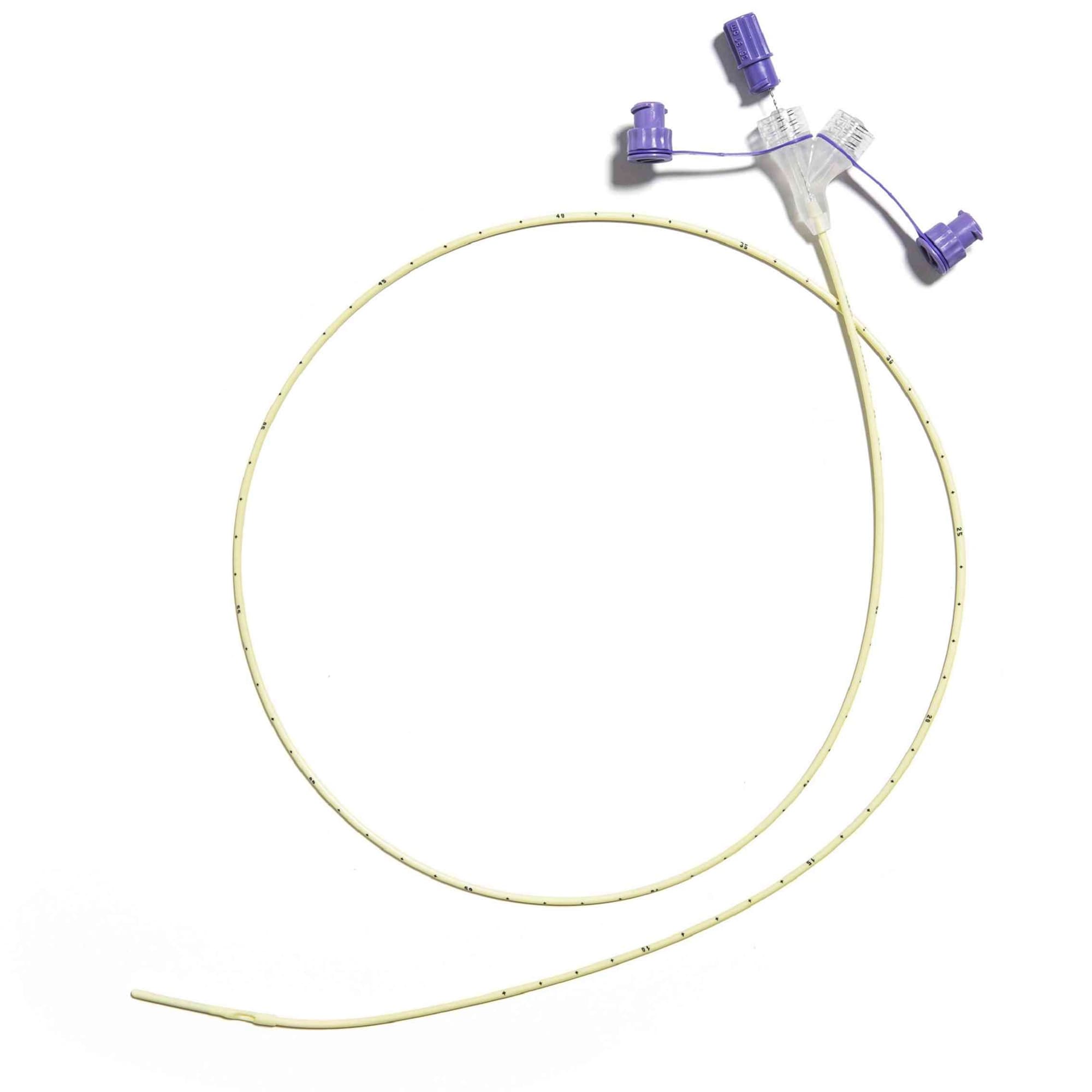
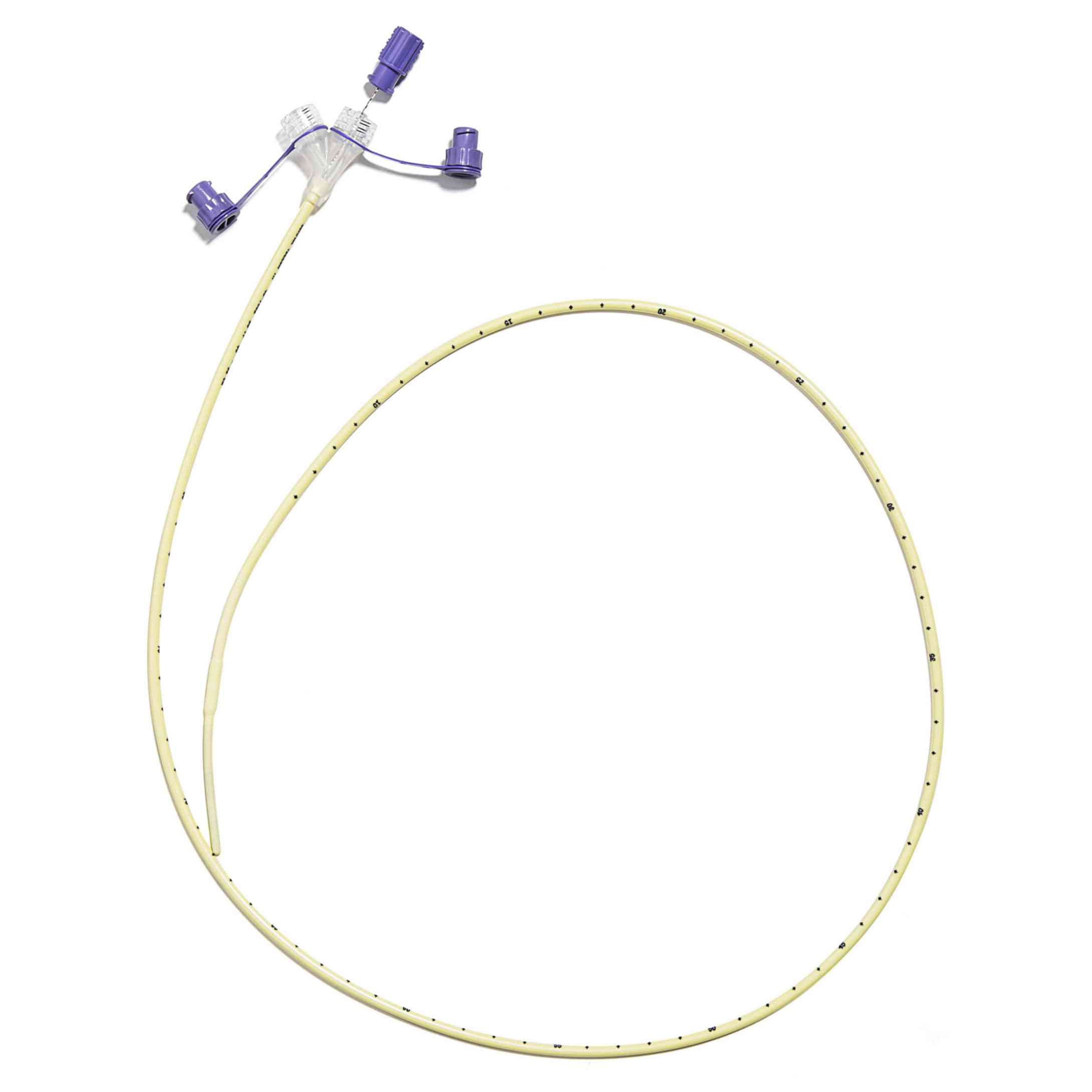
Corflo Nasogastric Feeding Tube with ANTI-IV Connector, Non Enfit, 6 Fr, 36" - Each
Earn Loyalty Points
+40Buy more to unlock extra rewards!
Description
Corflo Feeding Tube is designed for patients who require reliable intermittent or continuous enteral feeding through the nasogastric or nasoenteric route. Engineered for consistent performance, it supports safe delivery of nutrition and medications while maintaining a user-friendly design that helps enhance clinical workflow and patient comfort.
Features
- ENFit connector: No - Designed without an ENFit interface for facilities using traditional connection systems, ensuring compatibility with existing enteral setups.
- Sterilization: Non-sterile - Intended for clinical environments where sterile packaging is not required, supporting cost-effective and efficient supply management.
- Anti-IV connector: Yes - Equipped with an Anti-IV connector to help minimize the risk of misconnections, promoting safer enteral feeding and reducing cross-route errors.
Indications for Use: AVANOS CORFLO Feeding Tube is intended for intermittent or continuous nasogastric/nasoenteric feeding.
Device Lifetime: Validated for up to 30 days.
Tube Maintenance
- Follow facility protocol or clinician orders.
- Flush every 4 hours with up to 20 mL water (≤10 mL for infants/children) before/after meds or when feeding is interrupted.
- Monitor regularly; replace when clinically indicated.
Possible Complications
- Pneumothorax, GI perforation, aspiration, airway obstruction, tissue irritation/necrosis, allergic reaction, contamination, delayed diagnosis.
- Medication/nutrition misdose or delay; need for additional procedures.
MRI Safety
Weighted CORFLO tubes are MR Conditional (3T or less):
- Static field ≤3 Tesla
- Spatial gradient ≤720 Gauss/cm
- Whole-body SAR ≤3.0 W/kg
Warnings
- Do not lean the patient forward or extend the head/neck during insertion.
- Pre-measure length; never insert excess (risk of kinking/occlusion).
- Coughing may indicate tracheal placement - remove immediately if suspected.
- Confirm gastric placement before use. Resistance = remove tube.
- Never reinsert the stylet while the tube is in the patient.
- Avoid vigorous syringe force (risk of tube rupture).
Note: Crush medications finely and dilute well (no visible particles) to prevent clogging.
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
Corflo Feeding Tube is designed for patients who require reliable intermittent or continuous enteral feeding through the nasogastric or nasoenteric route. Engineered for consistent performance, it supports safe delivery of nutrition and medications while maintaining a user-friendly design that helps enhance clinical workflow and patient comfort.
Features
- ENFit connector: No - Designed without an ENFit interface for facilities using traditional connection systems, ensuring compatibility with existing enteral setups.
- Sterilization: Non-sterile - Intended for clinical environments where sterile packaging is not required, supporting cost-effective and efficient supply management.
- Anti-IV connector: Yes - Equipped with an Anti-IV connector to help minimize the risk of misconnections, promoting safer enteral feeding and reducing cross-route errors.
Indications for Use: AVANOS CORFLO Feeding Tube is intended for intermittent or continuous nasogastric/nasoenteric feeding.
Device Lifetime: Validated for up to 30 days.
Tube Maintenance
- Follow facility protocol or clinician orders.
- Flush every 4 hours with up to 20 mL water (≤10 mL for infants/children) before/after meds or when feeding is interrupted.
- Monitor regularly; replace when clinically indicated.
Possible Complications
- Pneumothorax, GI perforation, aspiration, airway obstruction, tissue irritation/necrosis, allergic reaction, contamination, delayed diagnosis.
- Medication/nutrition misdose or delay; need for additional procedures.
MRI Safety
Weighted CORFLO tubes are MR Conditional (3T or less):
- Static field ≤3 Tesla
- Spatial gradient ≤720 Gauss/cm
- Whole-body SAR ≤3.0 W/kg
Warnings
- Do not lean the patient forward or extend the head/neck during insertion.
- Pre-measure length; never insert excess (risk of kinking/occlusion).
- Coughing may indicate tracheal placement - remove immediately if suspected.
- Confirm gastric placement before use. Resistance = remove tube.
- Never reinsert the stylet while the tube is in the patient.
- Avoid vigorous syringe force (risk of tube rupture).
Note: Crush medications finely and dilute well (no visible particles) to prevent clogging.
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.