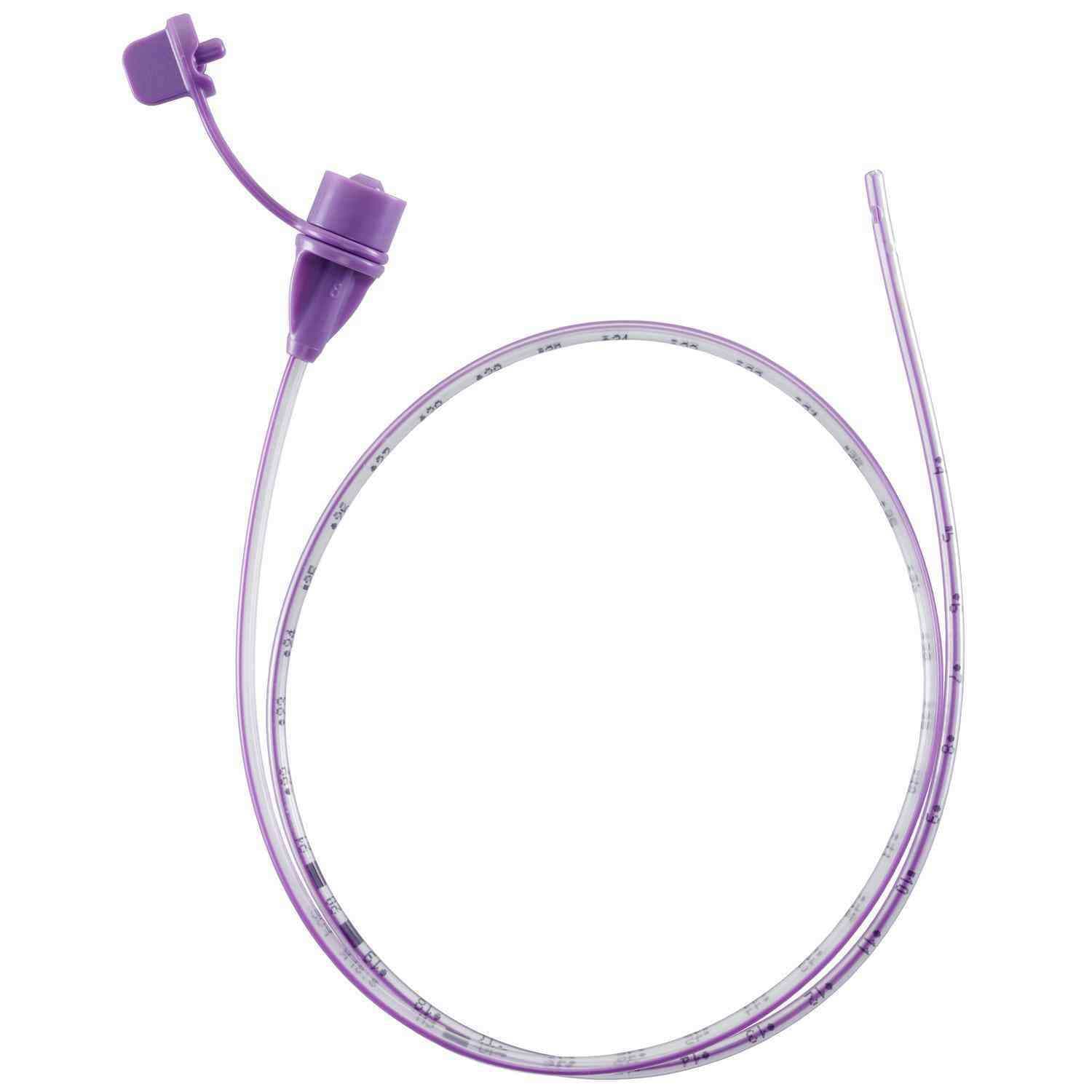
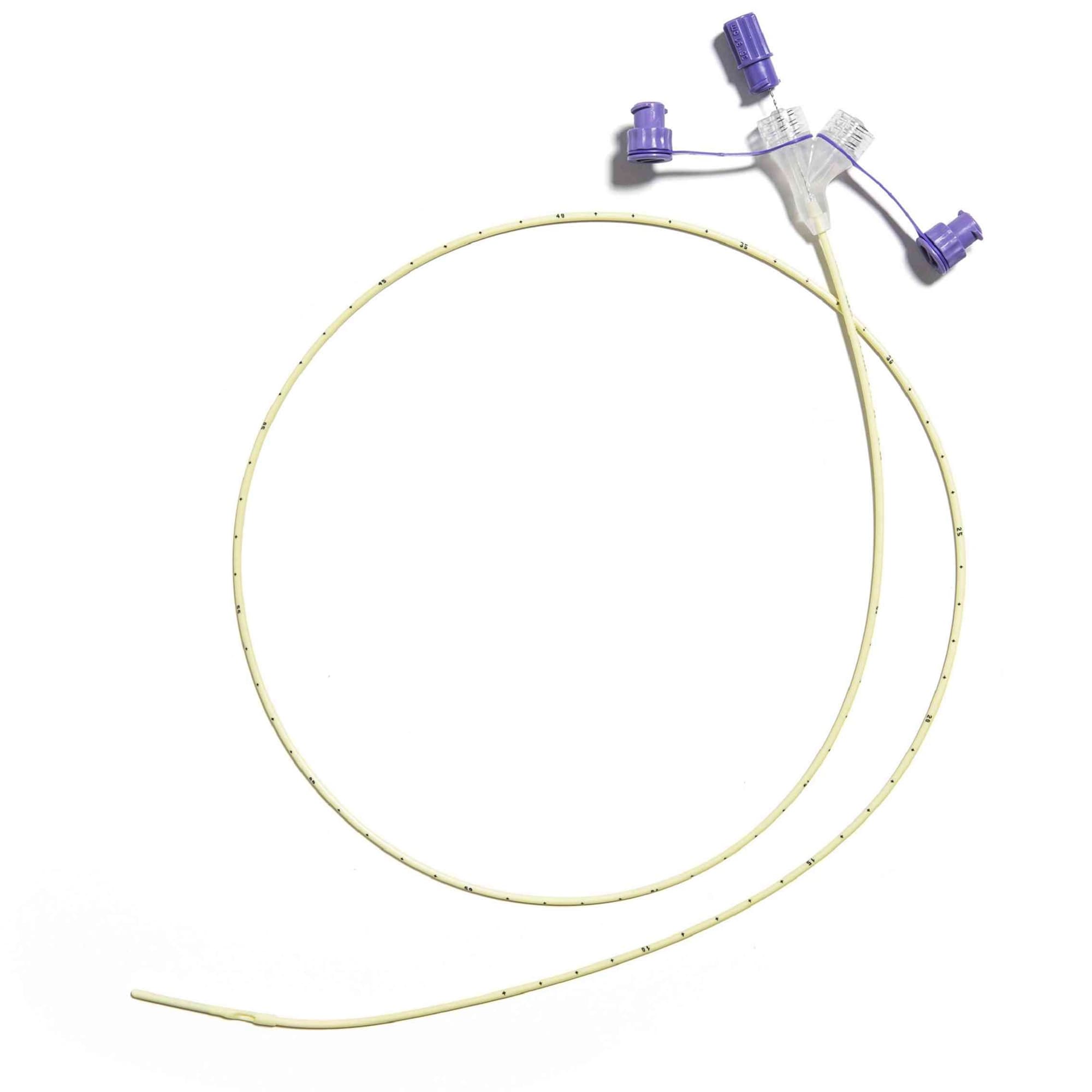
Corflo-Ultra Enfit Nasogastric Feeding Tube with 3g Weighted Tip
Starts From
Description
Corflo Feeding Tube is designed for patients who need either continuous or intermittent enteral nutrition delivered through the nasogastric or nasoenteric pathway. It provides a reliable solution for safe and effective nutritional support across various clinical settings.
Features
- Sterilization: Supplied non-sterile for flexible use depending on facility protocols.
- Bolus Type: Features an anti-clog design to help maintain consistent flow and reduce blockages.
- Tip Weight Type: Incorporates a weighted tip to support smooth placement and enhanced tube stability.
- ENFit Connector: Equipped with an ENFit connection for improved patient safety and compatibility with ENFit-compliant devices.
Indications for Use: The AVANOS CORFLO Feeding Tube is intended for use in those patients who require intermittent or continuous tube feedings via the nasogastric or nasoenteric route.
Device Lifetime: Device has been validated for use up to 30 days.
Tube Maintenance
- Follow your institution/facility/hospital protocol or clinician’s order.
- It is recommended that the tube be irrigated every 4 hours with up to 20 ml of water (up to 10 ml for infants or children) before and after medication administration or when feeding formula is interrupted.
- The feeding tube should be monitored, regularly assessed, and replaced when clinically indicated based on functionality and patient condition.
Complications
Infrequently occurring factors as a result of use or misuse of any feeding tube may include:
- Pneumothorax, GI perforation, Aspiration, Airway obstruction, Tissue irritation or necrosis, Allergic reaction, Contamination, and Delay in diagnosis.
- Delay or misdose of medication or nutrition and related complications or the need for additional medical procedures.
MRI Safety Information
Non-clinical testing demonstrated that the weighted CORFLO Enteral Feeding Tubes are MR Conditional. A patient with this device can be scanned safely immediately after placement under the following conditions:
- Static magnetic field of 3-Tesla or less
- Spatial gradient magnetic field of 720-Gauss/cm or less
- MRI system reported a whole body averaged SAR of 3.0-W/kg (i.e., associated with a calorimetry measured value of 2.8- W/kg)
For more Info, you can refer to the PDF
Warnings:
- The patient should not lean forward, nor should the head and neck be extended.
- Pre-measurement of tubing length is essential. Do not insert excess. Occlusion may result from the kinking of the tube.
- Coughing may indicate passage of the tube into the trachea. If tracheal passage is suspected, remove the tube. Absence of coughing does not confirm tube placement in the stomach. If resistance is encountered, immediately remove the tube. Notify clinician. Care should be taken if any type of endotracheal device is in place, as it may guide the feeding tube into the trachea. Misplacement of the feeding tube into the trachea or lungs may result in serious injury.
- Tube position in the stomach must be confirmed before flushing and use.
- Never reinsert the stylet when the tube is in the patient.
- Vigorous syringe force should not be used to irrigate, administer liquids, or unblock the tube.
Note: When administering crushed medicine, be sure to crush it finely and dilute it as appropriate (until there are no visible particles) to ensure the solution is of a low enough viscosity to avoid clogging.
Specifications
| Brand | CORFLO-ULTRA NG |
| Manufacturer | Avanos Medical |
| Application | Nasogastric Feeding Tube |
| Bolus Type | ANTI-CLOG |
| ENFit Connector | Yes |
| Primary Product Color | Yellow |
| Radiopaque | Yes |
| Radiopaque Stripe | No |
| Sterile | False |
| Tip Weight (g) | 3 |
| Tip Weight Type | Weighted |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
Corflo Feeding Tube is designed for patients who need either continuous or intermittent enteral nutrition delivered through the nasogastric or nasoenteric pathway. It provides a reliable solution for safe and effective nutritional support across various clinical settings.
Features
- Sterilization: Supplied non-sterile for flexible use depending on facility protocols.
- Bolus Type: Features an anti-clog design to help maintain consistent flow and reduce blockages.
- Tip Weight Type: Incorporates a weighted tip to support smooth placement and enhanced tube stability.
- ENFit Connector: Equipped with an ENFit connection for improved patient safety and compatibility with ENFit-compliant devices.
Indications for Use: The AVANOS CORFLO Feeding Tube is intended for use in those patients who require intermittent or continuous tube feedings via the nasogastric or nasoenteric route.
Device Lifetime: Device has been validated for use up to 30 days.
Tube Maintenance
- Follow your institution/facility/hospital protocol or clinician’s order.
- It is recommended that the tube be irrigated every 4 hours with up to 20 ml of water (up to 10 ml for infants or children) before and after medication administration or when feeding formula is interrupted.
- The feeding tube should be monitored, regularly assessed, and replaced when clinically indicated based on functionality and patient condition.
Complications
Infrequently occurring factors as a result of use or misuse of any feeding tube may include:
- Pneumothorax, GI perforation, Aspiration, Airway obstruction, Tissue irritation or necrosis, Allergic reaction, Contamination, and Delay in diagnosis.
- Delay or misdose of medication or nutrition and related complications or the need for additional medical procedures.
MRI Safety Information
Non-clinical testing demonstrated that the weighted CORFLO Enteral Feeding Tubes are MR Conditional. A patient with this device can be scanned safely immediately after placement under the following conditions:
- Static magnetic field of 3-Tesla or less
- Spatial gradient magnetic field of 720-Gauss/cm or less
- MRI system reported a whole body averaged SAR of 3.0-W/kg (i.e., associated with a calorimetry measured value of 2.8- W/kg)
For more Info, you can refer to the PDF
Warnings:
- The patient should not lean forward, nor should the head and neck be extended.
- Pre-measurement of tubing length is essential. Do not insert excess. Occlusion may result from the kinking of the tube.
- Coughing may indicate passage of the tube into the trachea. If tracheal passage is suspected, remove the tube. Absence of coughing does not confirm tube placement in the stomach. If resistance is encountered, immediately remove the tube. Notify clinician. Care should be taken if any type of endotracheal device is in place, as it may guide the feeding tube into the trachea. Misplacement of the feeding tube into the trachea or lungs may result in serious injury.
- Tube position in the stomach must be confirmed before flushing and use.
- Never reinsert the stylet when the tube is in the patient.
- Vigorous syringe force should not be used to irrigate, administer liquids, or unblock the tube.
Note: When administering crushed medicine, be sure to crush it finely and dilute it as appropriate (until there are no visible particles) to ensure the solution is of a low enough viscosity to avoid clogging.
Specifications
| Brand | CORFLO-ULTRA NG |
| Manufacturer | Avanos Medical |
| Application | Nasogastric Feeding Tube |
| Bolus Type | ANTI-CLOG |
| ENFit Connector | Yes |
| Primary Product Color | Yellow |
| Radiopaque | Yes |
| Radiopaque Stripe | No |
| Sterile | False |
| Tip Weight (g) | 3 |
| Tip Weight Type | Weighted |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Starts From